Session Information
Date: Sunday, November 7, 2021
Title: Epidemiology & Public Health Poster II: Inflammatory Arthritis – RA, SpA, & Gout (0560–0593)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: The Department of Veterans Affairs (DVA) provides comprehensive medical care at minimal or no cost to 9 million veterans annually through 170 medical centers and 1074 outpatient clinics across the United States. In 1999, the DVA established a national, fully integrated electronic health record (EHR), which now includes approximately 24 million veterans. However, few studies have used Veterans Affairs (VA) EHR data to examine the validity of diagnoses of RA. We developed a validated, national database of patients with RA who received VA care since the introduction of International Classification of Diseases, tenth revision (ICD-10) coding in 2015. This Veterans Affairs National Rheumatoid Arthritis Database (VANRAD) will provide infrastructure for retrospective and prospective research to address the ‘real-world’ care of patients with RA.
Methods: Patients with the following criteria were identified from the VA EHR as of October 2, 2020: (a) ≥1 ICD-10 diagnosis code of RA; (b) treatment with ≥1 DMARD; (c) ≥2 VA rheumatology clinic visits; and (d) ≥1 RF and/or anti-CCP (aCCP) antibody test result. From this group, 553 EHRs were randomly selected for review. The ‘gold standard’ for the diagnosis of RA was the treating rheumatologist’s diagnosis, documented in the EHR.
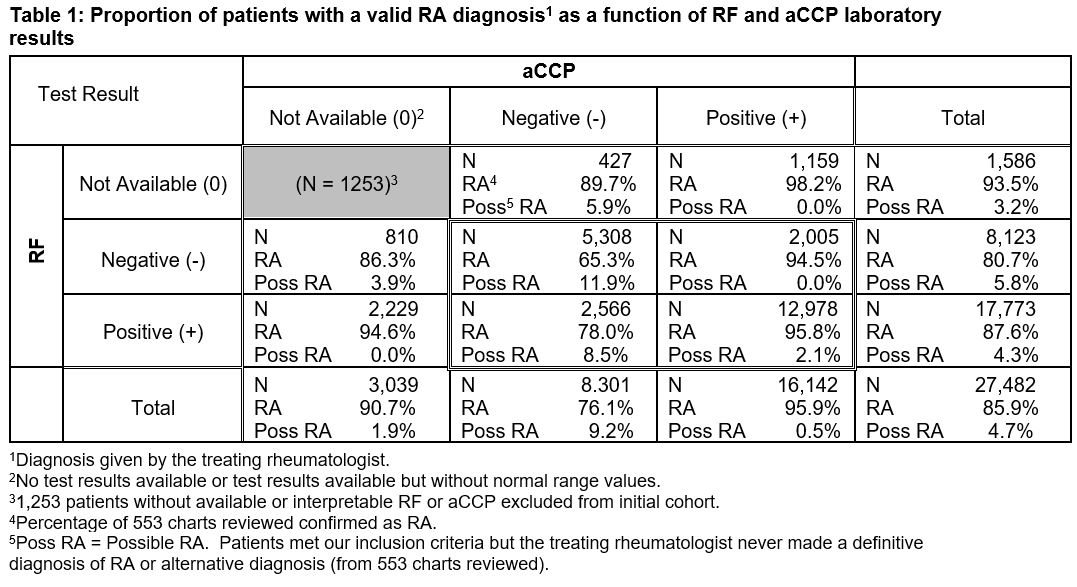
Results: A total of 27,482 patients met eligibility criteria. Sociodemographic characteristics were: 85.6% male; mean age of 69.7 years (y) (standard deviation [SD]=10.9 y; range=21.9 y to 100.5 y); 76.4% white, 17.0% African American; and mean VA care for 14.1 y (SD=5.3 y, range=0.04 y to 20.0 y).
For patients with ≥1 RF or aCCP test, the positive predictive value (PPV) for RA ranged from 65.3% (RF-/aCCP-) to 95.8% (RF+/aCCP+); rheumatologists’ likelihood of a ‘possible’ diagnosis was higher if the aCCP test result was negative or not available (Table 1). Excluding patients with a second rheumatologic diagnosis did not improve PPV results (data not shown).
The percentage of RA-confirmed patients with 1 test not available, and whose complementary test was negative (RF0/aCCP- or RF-/aCCP0), was greater than the percentage of patients for whom both tests were negative (RF-/aCCP-). This suggests our data extraction methods may have been incomplete or that unidentified bias may have been present, and warrants further study.
Conclusion: Our methodology for constructing an RA database by selecting patients with ≥2 rheumatology clinic visits, an ICD-10 diagnosis of RA, treatment with ≥1 DMARD, and a minimum of 1 RF or aCCP test result has high positive predictive value for RA. Positive RF and aCCP test results were strong predictors of rheumatologists’ diagnostic certainty for an RA diagnosis. Thus, the VANRAD and the associated EHR provide opportunity for a wide range of retrospective observational and prospective longitudinal studies based on ‘real-world’ patient care.
References: Ng B, et al. Arthritis Care Res 2012;64:1490-6; Hanly JG, et al. Open Access Rheumatol 2015;7:69-75.
Original abstract © 2020 EULAR/BMJ
To cite this abstract in AMA style:
Joseph A, Yanagida J, Huang X, Ranganathan P, Laurie M, Xian H, Eisen S. Construction of the Veterans Affairs National Rheumatoid Arthritis Database (VANRAD) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/construction-of-the-veterans-affairs-national-rheumatoid-arthritis-database-vanrad/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/construction-of-the-veterans-affairs-national-rheumatoid-arthritis-database-vanrad/