Session Information
Date: Sunday, October 21, 2018
Title: 3S085 ACR Abstract: Osteoarthritis & Joint Biology–Basic Science (846–850)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose:
Macrophage infiltration in synovium (SM) and intraarticular fat pads (FP) is common in Osteoarthritis (OA), and can contribute to catabolic cytokine and protease production as well as symptoms. However, whether macrophages are targets for therapy in OA is unclear, as macrophages can also promote tissue repair. The purpose of this study is to characterize the timeline and subtypes of macrophages in SM and FP in a commonly used murine model of post-traumatic OA.
Methods:
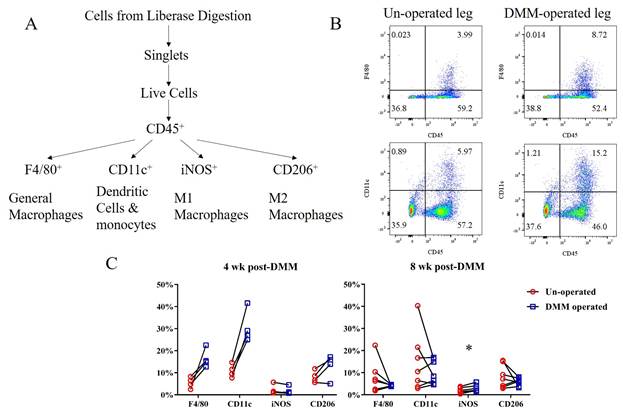
C57BL/6 male mice (10-12 wks old) were subjected to destabilization of medial meniscus (DMM) on the right leg. For gene expression analysis, groups of mice were sacrificed pre-DMM (baseline), and post-DMM at 1, 2, 4, 8 and 16 weeks. Anterior SM and FP (combined) was dissected. Tissues were pooled from 4-5 mice per sample to obtain adequate mRNA, cDNA synthesized by routine methods, and CD68 (a pan-macrophage marker) mRNA transcript number quantified using the QX200TM Droplet Digital PCR System (BioRad). Additional mice were sacrificed 4 and 8 weeks post-surgery and SM/FP dissected for cellular analysis. Tissues from 4 knees were pooled, cells isolated enzymatically, and stained with the Live/Dead™ Fixable Violet Dead Cell Stain Kit (Invitrogen) and the following antibodies: CD45-PerCP Cy5.5, CD11c-Super Bright 645, F4/80-APC, iNOS-Alexa Fluor 488, CD206-PE. Multicolor flow cytometry was performed and data analyzed with FlowJo software (Version 10). Gating strategy is outlined in Fig 1A, and cells were expressed as percent of the CD45+ population.
Results:
CD68 mRNA level increased in DMM-operated limbs compared to preoperative levels, peaking at 4 weeks (21.61±3.02, Baseline: 2.24±0.42). Levels remained significantly elevated compared to the un-operated limb up to 16 weeks (DMM: 4.77±0.31, contralateral: 0.90±0.26). Percentages of F4/80+ macrophages and CD11c+ cells (expressed by murine monocytes and dendritic cells) in SM/FP were increased at 4 weeks post- DMM but not at 8 weeks (Fig 1B & C). iNOS+ cells (primarily expressed on M1 macrophages) were slightly elevated compared to the un-operated side only at 8 weeks (p=0.02). Percentage of CD206+ cells (expressed on M2 macrophages) were similar in DMM and un-operated knees at either 4 or 8 weeks (Fig 1C).
Conclusion:
Changes in macrophage populations can be detected in SM/FP tissues after DMM surgery by both gene expression and flow cytometry. CD68 gene expression was most pronounced early at 4 weeks, and paralleled changes in F4/80+ and CD11c+ cells. Flow cytometry of M1 and M2 markers suggested a slight increase in iNOS+ (M1-type) cells at 8 weeks and CD206 at 4 weeks. Additional work is being pursued to further characterize macrophage phenotypes, and confirm gene expression seen at the chronic stage (16 weeks). These observations will be used to plan timing of interventions to better understand the pathologic roles of macrophage subtypes in OA.
To cite this abstract in AMA style:
Zhou C, Nguyen V, Sambamurthy N, Dodge M, Scanzello C. Constructing a Macrophage Infiltration Timeline in a Murine Model of Osteoarthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/constructing-a-macrophage-infiltration-timeline-in-a-murine-model-of-osteoarthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/constructing-a-macrophage-infiltration-timeline-in-a-murine-model-of-osteoarthritis/