Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Biomarkers play an important role in RA and can help guide treatment decisions. Previous studies have suggested differential treatment efficacy of abatacept (ABA) in patients with biomarker-seropositive RA,1–4 including differential treatment impact of ABA on ACR20/50/70 among patients with SeroPositive Early & Active RA (SPEAR) in historic randomized controlled trials (RCTs).5 In this study, we analyzed clinical outcomes among patients with SPEAR and non-SPEAR to understand the differential treatment impact of ABA on each ACR core measure.
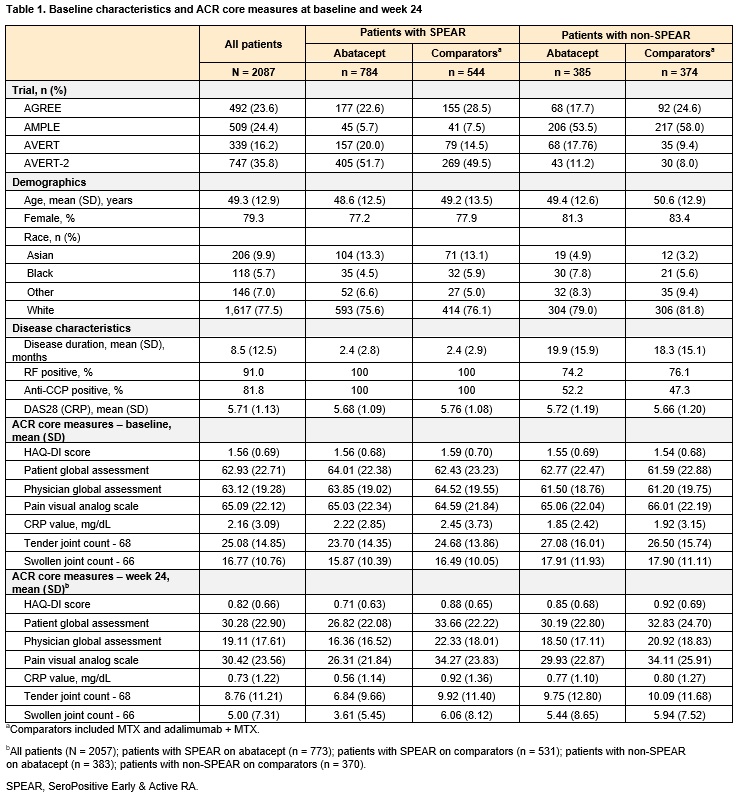
Methods: Individual patient data (IPD) from 2087 patients in 4 phase 3, early RA ABA RCTs (AGREE [NCT00122382], AMPLE [NCT00929864], AVERT [NCT01142726], AVERT-2 [NCT02504268]) were pooled. Patients were classified as SPEAR at baseline if they: 1) were RF+ and > 3 times the upper limit of normal on an anti-CCP test (ACPA+), 2) had a disease duration < 12 months, and 3) had DAS28 (CRP) > 3.2. Patients were treated with either ABA (monotherapy or with MTX) or a comparator (MTX or adalimumab + MTX). Outcomes of interest included mean change from baseline to week 24 in ACR core measures (e.g., HAQ-DI score, CRP value, patient global assessment). IPD mixed-effects regressions for each outcome used trial fixed effects, baseline measures of the outcome, and SPEAR status as covariates in the ABA-treated population. In the analysis of the full population, treatment type was added as well as an interaction term of treatment type and SPEAR status. Sensitivity analyses using an alternative SPEAR definition restricted to criterion 1 were also conducted.
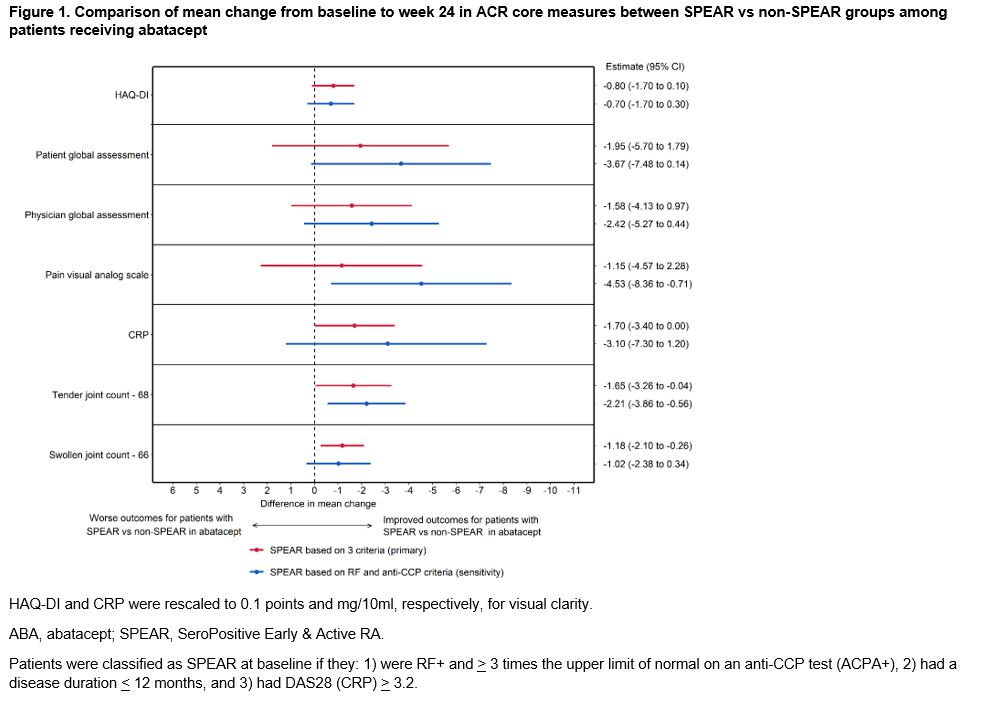
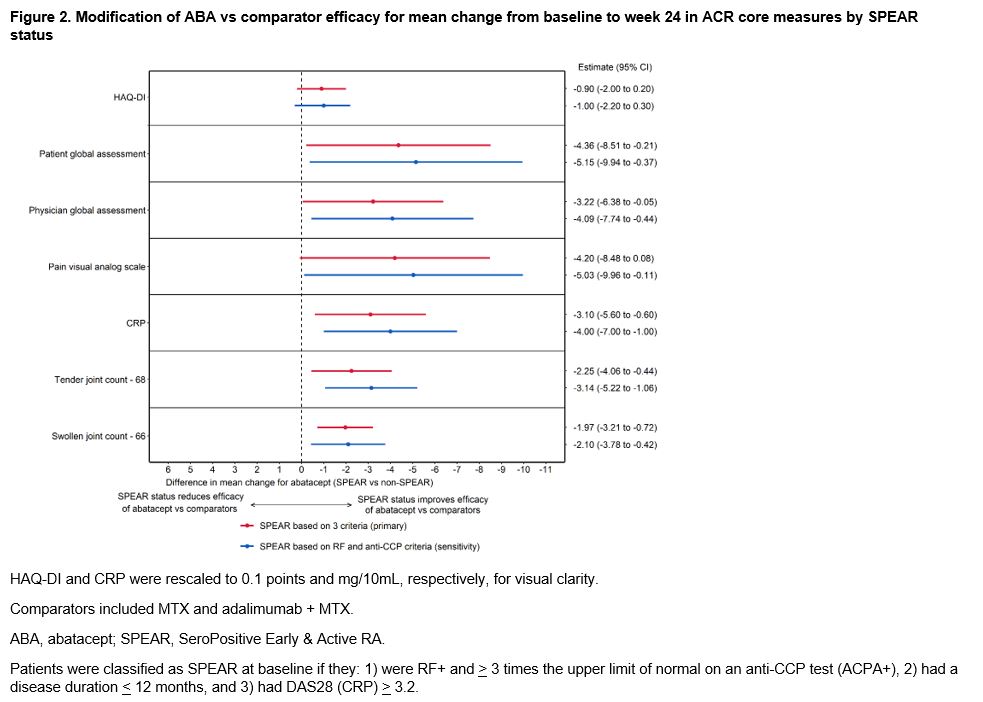
Results: A total of 1328 (64%) SPEAR and 759 (36%) non-SPEAR were included. For patients with non-SPEAR, around half were RF+ and three-quarters were ACPA+, while DAS28 (CRP) was high for all patients (Table 1). Among ABA-treated patients, outcomes were more favorable for patients with SPEAR compared to patients with non-SPEAR across all outcomes, either statistically or directionally (Figure 1). The differences in outcomes between patients with SPEAR vs non-SPEAR were larger for ABA than for comparators across all outcomes with the exceptions of HAQ-DI and pain, where the differences continued to be directionally favorable for ABA (Figure 2). The results were consistent in the sensitivity analysis.
Conclusion: This study compared clinical outcomes of patients with enriched autoantibody biomarkers and early RA (SPEAR) to patients with non-SPEAR and found a differential treatment impact of abatacept across all ACR core measures in the RCTs analyzed. These findings corroborate prior studies and further suggest that the differential treatment impact of abatacept is not limited to a subset of core measures but affects all dimensions including laboratory values, joint counts, functional status, patient reported outcomes, and physician global assessment.
References:
1. Buckner J, et al. Arthritis Rheumatol 2019;71(Suppl 10):abstract 1424.
2. Huizinga T, et al. Ann Rheum Dis 2015;74:234–235.
3. Harrold LR, et al. Rheumatol Ther 2019;6:217–230.
4. Sokolove J, et al. Ann Rheum Dis 2016;75:709–714.
5. Michaud K, et al. Ann Rheum Dis POS0474, in press.
Editorial support: Danielle Johnson (Caudex), funded by Bristol Myers Squibb
To cite this abstract in AMA style:
Conaghan P, Park S, Fillbrunn M, Lozenski K, Khaychuk V, Michaud K, Swallow E, Lane H, Nguyen H, Pope J. Consistent Impact of Autoantibody Enrichment Across All ACR Core Measures in Early Rheumatoid Arthritis Treated with Abatacept: Data from a Large Pooled Analysis of 4 Randomized Controlled Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/consistent-impact-of-autoantibody-enrichment-across-all-acr-core-measures-in-early-rheumatoid-arthritis-treated-with-abatacept-data-from-a-large-pooled-analysis-of-4-randomized-controlled-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consistent-impact-of-autoantibody-enrichment-across-all-acr-core-measures-in-early-rheumatoid-arthritis-treated-with-abatacept-data-from-a-large-pooled-analysis-of-4-randomized-controlled-trials/