Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: The nearly invariant finding of anti-SSA/Ro52/60kD autoantibodies in pregnancies complicated by cardiac neonatal lupus (cardiac-NL), which manifests as fetal atrioventricular block and endocardial fibroelastosis (EFE), supports these autoantibodies in the pathogenesis. The placental neonatal Fc receptor (FcRn) is responsible for transporting IgGs into the fetal circulation, and blocking FcRn has shown promise in treating fetal hemolytic disease. Application of this therapeutic strategy to prevent cardiac-NL is challenged by the absence of studies demonstrating the presence of anti-Ro antibodies in the developing fetus during the critical period of cardiac vulnerability between 18-25 gestational weeks. At this age, FcRn syncytiotrophoblast expression is incomplete, resulting in limited IgG transport. Accordingly, anti-Ro levels were measured in blood and serosal fluids of 2nd trimester fetuses dying with cardiac-NL to more precisely implicate these autoantibodies as causative.
Methods: Fluids (serial dilutions 1:1,000 – 1,100,000) were evaluated for IgG and IgM titers of anti-Ro antibodies using a research ELISA. For IgG, results are considered positive at 321 ELISA units (EU) for 52kD Ro and 75 EU for 60kD Ro (mean +2 SD of values for sera from 34 healthy women during normal pregnancies). Based on evaluation of > 100 cardiac-NL mothers, a threshold risk level of 1,000 EU for either Ro specificity has been previously published.
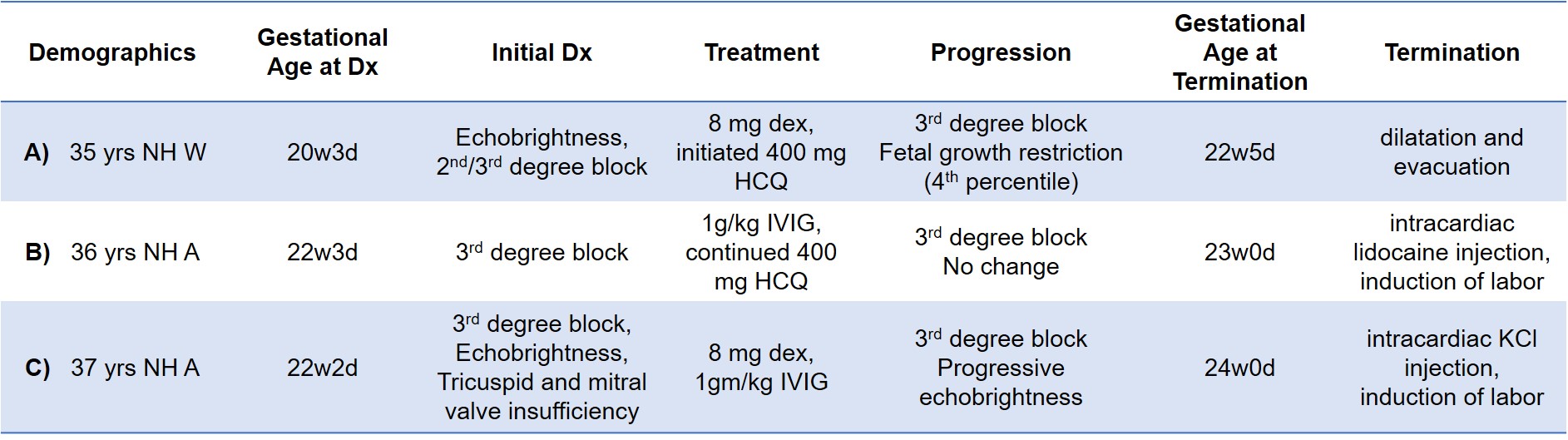
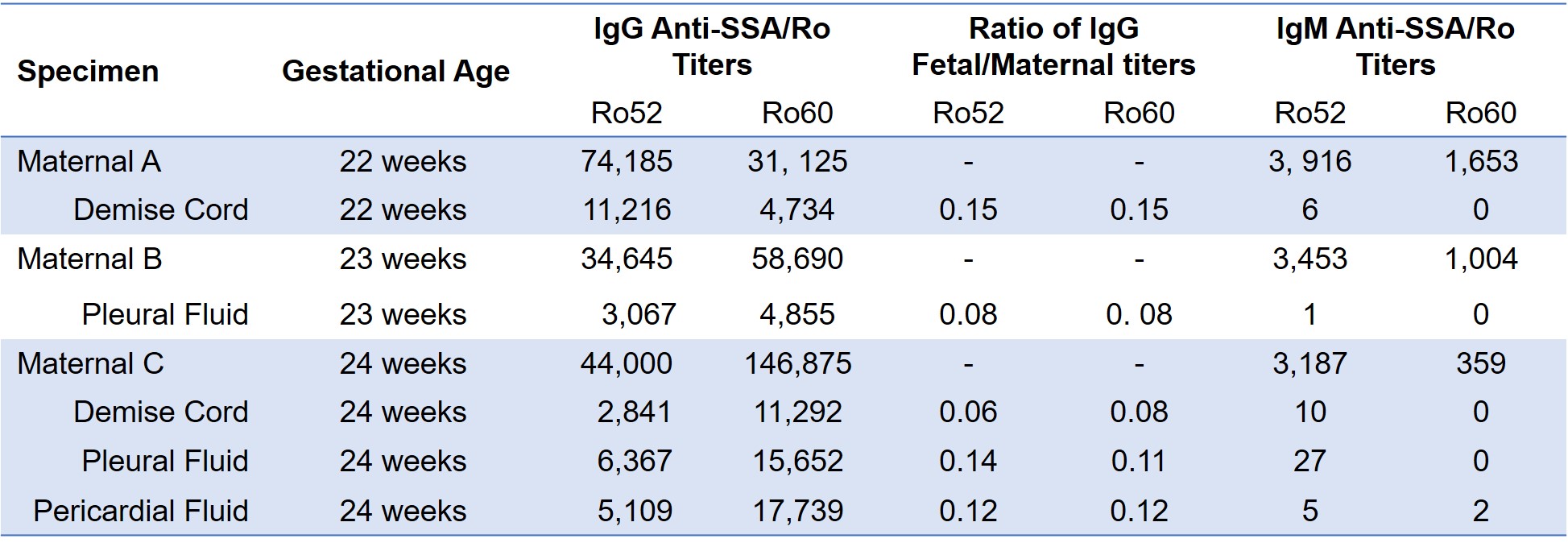
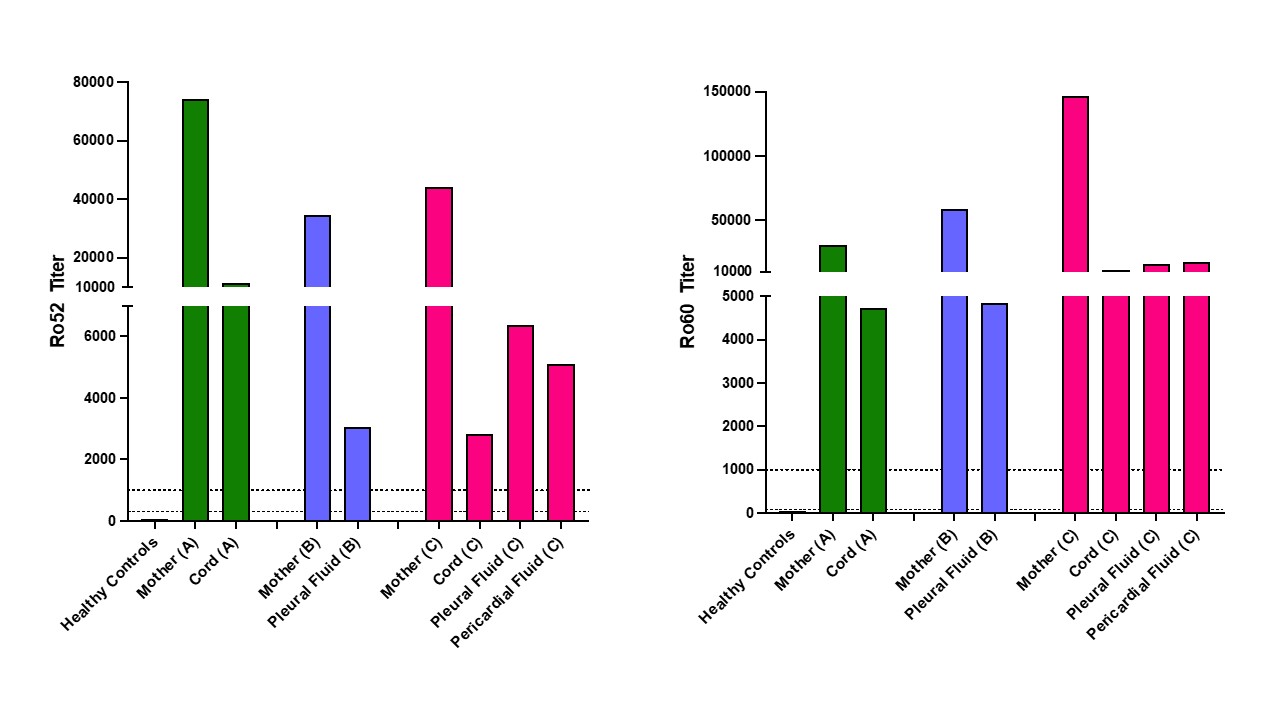
Results: Fluids from 3 terminated fetuses with cardiac-NL and matched maternal blood from the 2nd trimester were evaluated. All fetuses were diagnosed with advanced block in the 2nd trimester and treated with dexamethasone, IVIG, or both (Table 1). Only one pregnant patient was aware of anti-Ro and was taking hydroxychloroquine. Two fetuses showed progressive disease despite treatment prompting termination and one remained stable and terminated at parental request. Autopsy from one heart showed extensive EFE and multinucleated giant cells (fetus C). Maternal antibody reactivities to both Ro52 and 60 were in the top quartile of titers reported previously from 413 anti-Ro pregnancies. Levels of fetal IgG anti-Ro52 and 60 in the umbilical cord, pleural and pericardial space all exceeded the previously identified threshold level for risk of cardiac-NL (Table 2, Figure 1). In fetus C, levels were higher in serosal spaces than cord blood. Overall, fetal titers reached up to 15% of maternal levels for both reactivities and always matched the higher anti-Ro specificity in the pregnant patient. No IgM anti-Ro52 or 60 (FcRn does not bind IgM) were detected despite being present in the maternal circulation, confirming that the fetal anti-Ro antibodies were the consequence of placental transport and not inadvertent maternal contamination during evacuation or delivery (Table 2).
Conclusion: Despite limited FcRn transport, maternal anti-Ro52/60 autoantibodies of IgG isotype, not IgM, are present in the 2nd trimester fetus with levels above the threshold for high risk. Analogous to the encouraging early results in antibody mediated fetal hemolytic disease, consideration of “no antibody no disease” supports the approach of FcRn as a druggable target in the prevention of cardiac-NL.
NH = non-Hispanic, W = White, A = Asian, dex = dexamethasone, HCQ = hydroxychloroquine
To cite this abstract in AMA style:
Fraser N, Masson M, Clancy R, Carlucci P, Izmirly P, Sachan N, Brandt J, Thomas K, Fox M, Phoon C, Ludomirsky A, Srinivasan R, Lam G, Cuneo B, Buyon J. Confirmation of Second Trimester Trophoblast Transport of Maternal Anti-SSA/Ro52 and 60kD Autoantibodies in Cardiac Neonatal Lupus: Implications for FcRn Blockade [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/confirmation-of-second-trimester-trophoblast-transport-of-maternal-anti-ssa-ro52-and-60kd-autoantibodies-in-cardiac-neonatal-lupus-implications-for-fcrn-blockade/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/confirmation-of-second-trimester-trophoblast-transport-of-maternal-anti-ssa-ro52-and-60kd-autoantibodies-in-cardiac-neonatal-lupus-implications-for-fcrn-blockade/