Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Sprifermin is under investigation as a potential disease-modifying osteoarthritis drug (DMOAD). 2-yr results from the FORWARD study showed significant dose-dependent modification of cartilage thickness in the total femorotibial joint (TFTJ), medial and lateral femorotibial joints (MFTC, LFTC), and central medial and lateral TFTJ subregions, by quantitative (q)MRI (Hochberg et al. ACR 2017).
This post hoc analysis aimed to determine whether qMRI findings from FORWARD (manual segmentation) could be reproduced in the same cartilage regions using an independent method (automated segmentation), on the same dataset/time period.
Methods: Pts were randomized 1:1:1:1:1 to: sprifermin 100 µg q6mo; 100 µg q12mo; 30 µg q6mo; 30 µg q12mo; and placebo (n=110/110/111/110/108). Cartilage thickness was assessed at baseline and 6, 12, 18, and 24 months using 1.5- or 3-Tesla MRI images, analysed manually. The same images were analysed by automated cartilage segmentation using active appearance models, a supervised machine learning method, to produce maps of cartilage thickness for weight-bearing femoral and tibial cartilage surfaces, subdivided into anatomical masks. Results were blinded for treatment and timepoint for both methods. No statistical comparisons between methods were conducted.
Endpoints were change from baseline in: 1) cartilage thickness in the TFTJ, MFTC and LFTC, using regions duplicated based on published data; 2) cartilage thickness in the central subregion of the medial and lateral tibia and femur (cMT, cMF, cLT, cLF [conventions used by the automated analysis investigators]). As in previous analyses, treatment effect was assessed by observed changes and adjusted using repeated ANCOVA on change from baseline, including treatment group, timepoint, and country as fixed factors, baseline value as covariate and treatment by timepoint as interaction.
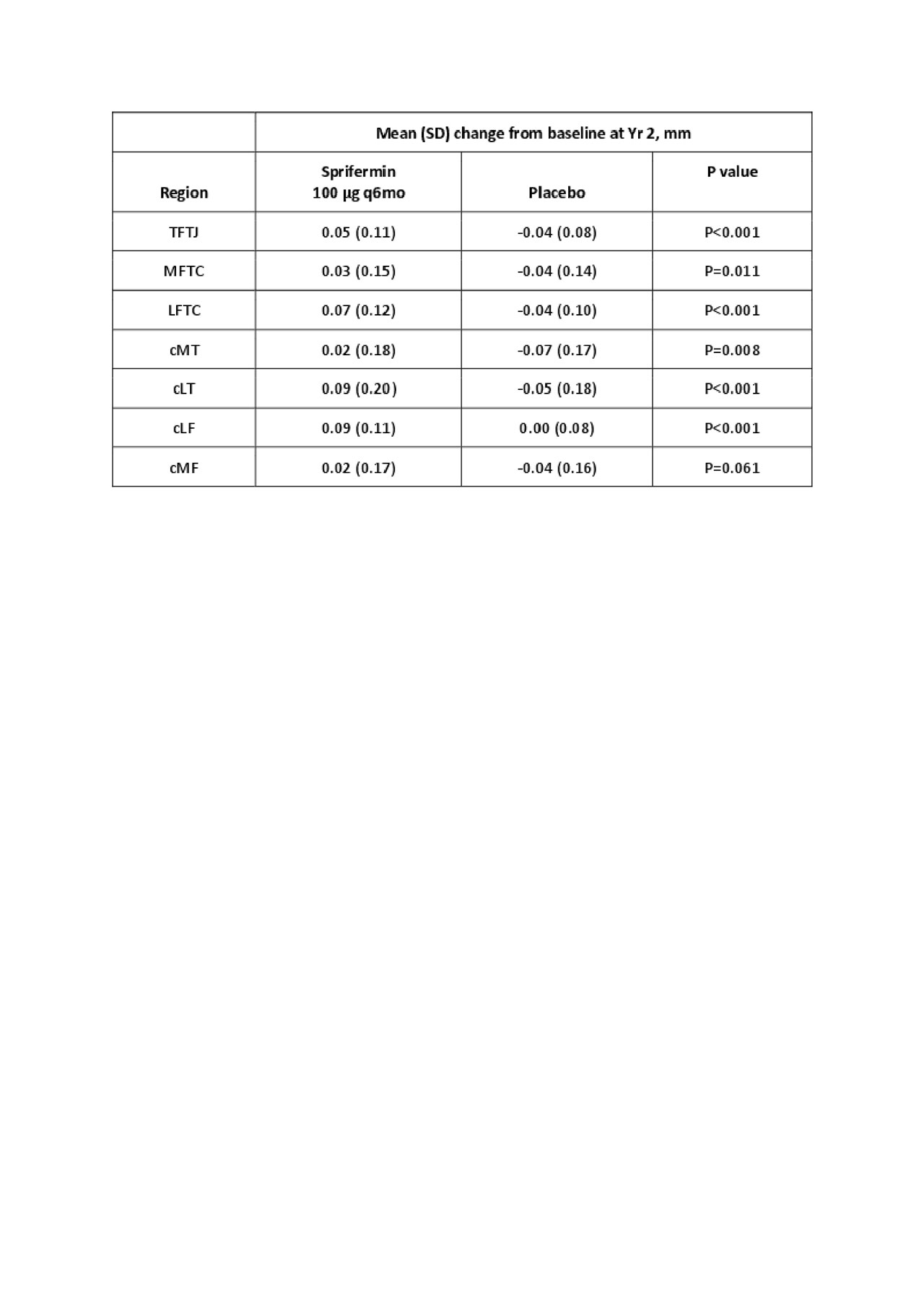
Results: Based on automated segmentation, statistically significant, dose-dependent structural modification of cartilage thickness was observed over 2 yrs with sprifermin vs placebo for the TFTJ (overall treatment effect and dose response across all doses, both P< 0.001), MFTC (P=0.004 and P=0.044), and LFTC (both P< 0.001). Table 1 shows changes from baseline for sprifermin 100 µg q6mo and placebo. Statistically significant dose-dependent structural modification of cartilage over 2 yrs was observed for sprifermin vs placebo in the cMT (100 µg q6mo), cLT (100 µg q6mo, q12mo) and cLF (100 µg q6mo, q12mo). In the cMF, there was no treatment effect, but there was a linear trend for dose responsiveness. The results showed a consistent pattern to those obtained using manual segmentation.
Conclusion: Cartilage thickness assessed by automated segmentation provided a consistent pattern of structural modification in FORWARD compared with manual segmentation. This is the first time that two independent methods of image analysis have reached the same conclusions in an interventional DMOAD trial. The findings strengthen the conclusions that sprifermin modifies cartilage loss/structural progression in knee OA.
To cite this abstract in AMA style:
Brett A, Bowes M, Conaghan P, Ladel C, Kraines J, Guehring H, Moreau F, Eckstein F. Confirmation of Manual Cartilage Segmentation Findings by Automated Segmentation: Retrospective Analysis of MRI Images from a Sprifermin Phase II Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/confirmation-of-manual-cartilage-segmentation-findings-by-automated-segmentation-retrospective-analysis-of-mri-images-from-a-sprifermin-phase-ii-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/confirmation-of-manual-cartilage-segmentation-findings-by-automated-segmentation-retrospective-analysis-of-mri-images-from-a-sprifermin-phase-ii-study/