Session Information
Date: Sunday, October 26, 2025
Title: (0280–0305) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: RNA/protein immunoprecipitation (IP) assays remain the “gold standard” for myositis-specific autoantibody (MSA) detection. However, the requirements for large-scale cell culture and radioisotopes limit its availability to specialized laboratories. Clinical practice has shifted toward commercial enzyme-linked immunosorbent assay (ELISA) and multianalyte line blot assay (LBA), yet their reliability is inadequately established. Our study provides the comprehensive evaluation of concordance between two widely used commercial assays against IP assays, establishing evidence-based guidelines for MSA testing in clinical practice.
Methods: Sera from patients with idiopathic inflammatory myopathies (IIMs) were collected across the Asia-Pacific region. Anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ, anti-melanoma differentiation-associated gene 5 (MDA5), anti-Mi-2, and anti-transcriptional intermediary factor (TIF) 1-γ antibodies were centrally measured using commercial ELISA kits (MBL, Tokyo, Japan) and LBA systems (EuroImmun, Lübeck, Germany). Positive percentage agreement (PPA), negative percentage agreement (NPA), and Cohen’s kappa were calculated to assess concordance between the two assays. Discordant results were further validated using RNA/protein IP assays.
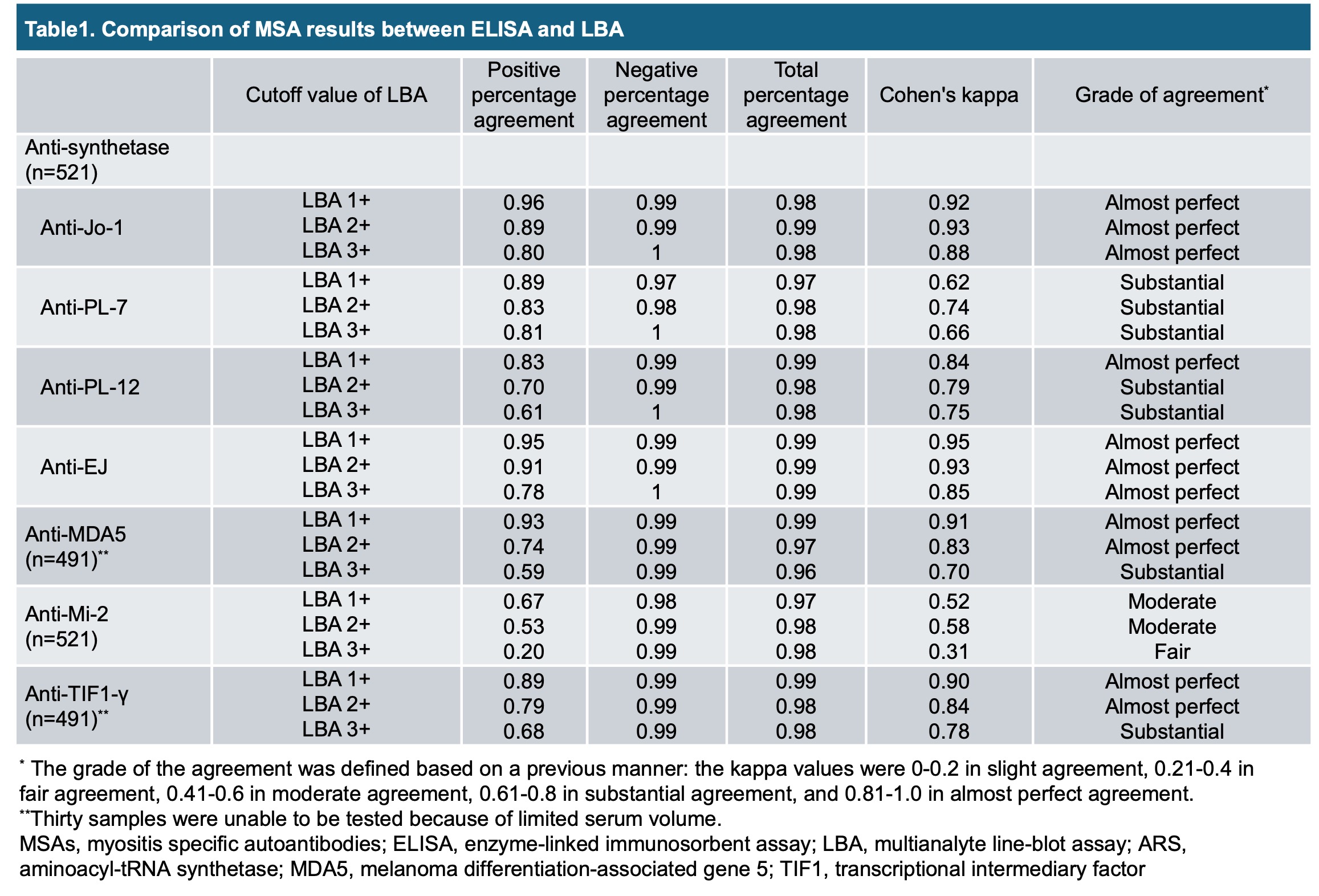
Results: A total of 521 sera from patients with dermatomyositis (DM; n = 180), amyopathic DM (n = 44), juvenile DM (n = 7), polymyositis or immune-mediated necrotizing myopathy (n = 197), inclusion body myositis (n = 57), and IIM-not-otherwise-specified (n = 36) were collected from Australia (n = 200), India (n = 83), Hong Kong (n = 35), Japan (n = 152), Malaysia (n = 21), Singapore (n = 20), and Thailand (n = 10). The highest PPA at the 1+ cutoff value for LBA was observed for anti-Jo-1 (0.96), followed by anti-EJ (0.95), anti-MDA5 (0.93), anti-TIF1-γ (0.89), anti-PL-7 (0.89), anti-PL-12 (0.83), and anti-Mi-2 (0.67). Kappa values indicated strong agreement for anti-Jo-1, anti-PL-12, anti-EJ, anti-MDA5, and anti-TIF1-γ, but lower agreement for anti-PL-7 and anti-Mi-2 (Table 1). PPA at the cutoff value of 2+ or 3+ for LBA was numerically lower for all MSAs, whereas NPA remained high in all (0.99-1). The validation analysis using IP assays in sera with discordant results between ELISA and LBA at the 1+ cutoff value revealed a high rate of false-positives for anti-PL-7 (7/9 samples) and anti-Mi-2 (6/6 samples) antibodies with LBA. The false-positive rates decreased at the 3+ cutoff value for anti-PL-7 (0/9) and anti-Mi-2 (1/6).
Conclusion: LBA demonstrates discordance in PPA based on the cutoff values across all MSAs. Clinicians should interpret LBA results with caution, particularly when evaluating antibodies to PL-7 and Mi-2.
To cite this abstract in AMA style:
Gono T, Limaye V, Gupta L, Agarwal V, So H, RAJA J, Fong W, Wangkaew S, Low A, Murakami A, Hasegawa K, Isayama T, Kuwana M. Concordance for myositis-specific autoantibody detection between commercial enzyme-linked immunosorbent assay and line blot assay: a multi-center study across the Asia-Pacific region [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/concordance-for-myositis-specific-autoantibody-detection-between-commercial-enzyme-linked-immunosorbent-assay-and-line-blot-assay-a-multi-center-study-across-the-asia-pacific-region/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/concordance-for-myositis-specific-autoantibody-detection-between-commercial-enzyme-linked-immunosorbent-assay-and-line-blot-assay-a-multi-center-study-across-the-asia-pacific-region/