Session Information
Date: Sunday, November 8, 2020
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: Several recent randomized controlled trials that used the validated ESSDAI as primary endpoint failed, partly explained by relatively large response rates in the placebo group. Since primary Sjögren’s Syndrome (pSS) is a very heterogeneous disease, a composite endpoint including multiple disease aspects may be more appropriate to demonstrate clinical efficacy. Therefore, our objective was to develop a composite endpoint for pSS based on expert opinion and analysis of trial data.
Methods: According to expert opinion, 5 items were found to be most relevant to assess the effect of treatment in pSS: systemic disease activity, patient-reported symptoms, tear gland, salivary gland and serological items. These items were tested using data from the randomized, double-blind, placebo-controlled ASAPIII trial at week 24.1 Cut-off points were based on ROC analysis to assess discrimination of effect between patients on abatacept (n=40) and placebo (n=39) and on expert opinion.
Results: The ‘Composite of Relevant Endpoints in Sjögren’s Syndrome’ (CRESS) consists of 5 complementary items. The definition of response for each item is presented in Table 1. For measuring systemic disease activity, ClinESSDAI2 is used as it showed higher discrimination compared to ESSDAI and because the biological domain is separately included in the CRESS. ROC analysis for absolute or relative change in ClinESSDAI showed no discrimination between treatment groups (AUC 0.534 and 0.565), therefore low disease activity (< 5) in ClinESSDAI was used. Patient-reported symptoms are measured with ESSPRI. As ROC analysis showed optimal cut-off points of -0.83 and -13.8% (AUC 0.621 and 0.629), the previously validated definition of ESSPRI response (≥15% or 1 point)3 was used. Tear and salivary gland items include both glandular function and imaging. Cut-off points were based on expert opinion, taking measurement variation into account. The CRESS can also be used without OSS and SGUS if these tests are not available, leaving Schirmer’s and UWS for assessing the tear and salivary gland item with similar response rates in both treatment groups. The serological item includes serum levels of RF-IgM and IgG. ROC analysis showed optimal cut-off points of -23% and -2.2% (AUC 0.861 and 0.615), respectively, which were set to ≥25% and ≥10% decrease to minimize placebo response due to natural variation. Total CRESS response was defined as response on ≥3 of the 5 items. The CRESS response per item for abatacept and placebo treatment groups is presented in Table 2. The total CRESS response rate was 65% vs. 18% (p< 0.001). As shown in Table 3, response to the 5 CRESS items is well-balanced.
Conclusion: The newly developed ‘Composite of Relevant Endpoints for Sjögren’s Syndrome’ (CRESS) enables discrimination between active treatment (abatacept) and placebo treatment in pSS patients. All items contribute equally to CRESS response. Validation analyses in independent, global, multi-center, placebo-controlled trials in pSS patients will be performed.
References
1van Nimwegen et al. Lancet Rheumatol. 2020;9913(19):1-11.
2Seror et al. Ann Rheum Dis. 2016;75(11):1945-50.
3Seror et al. Ann Rheum Dis. 2016 ;75(2) :382-9.
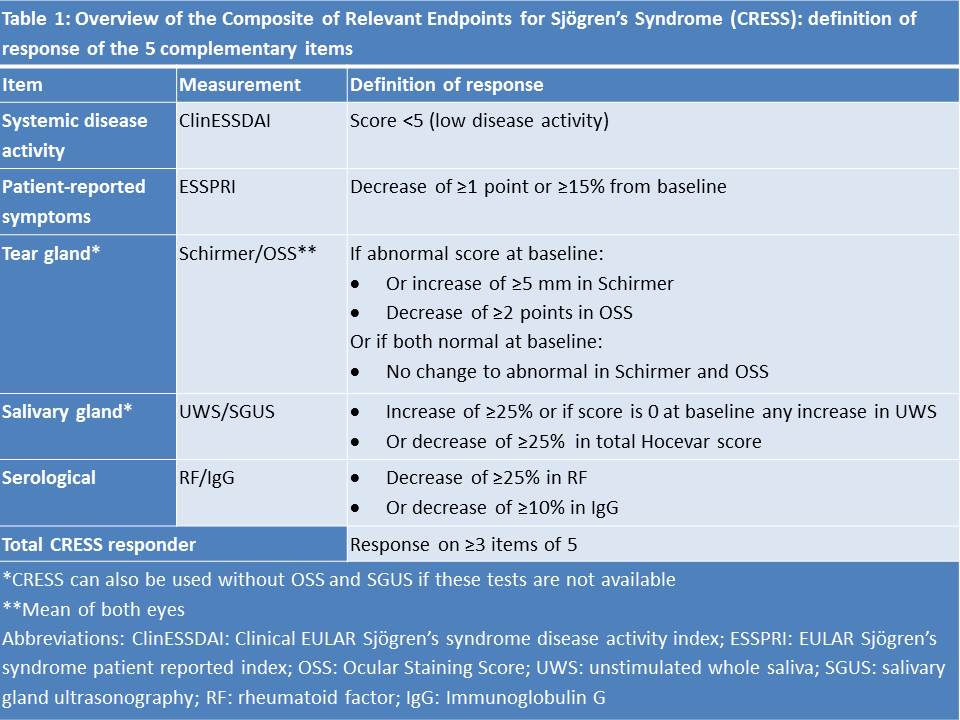 Table 1: Overview of the Composite of Relevant Endpoints for Sjögren’s Syndrome (CRESS): definition of response of the 5 complementary items
Table 1: Overview of the Composite of Relevant Endpoints for Sjögren’s Syndrome (CRESS): definition of response of the 5 complementary items
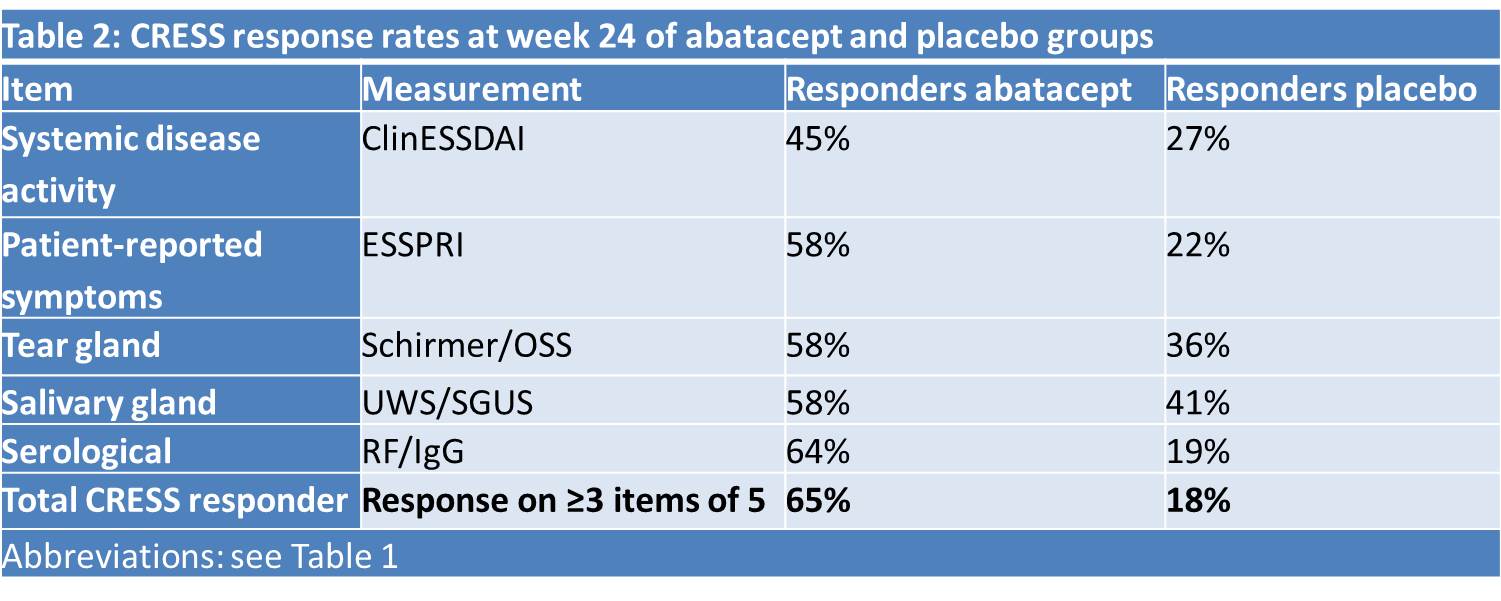 Table 2: CRESS response rates at week 24 of abatacept and placebo groups
Table 2: CRESS response rates at week 24 of abatacept and placebo groups
 Table 3: Response and non-response on individual items in CRESS responders
Table 3: Response and non-response on individual items in CRESS responders
To cite this abstract in AMA style:
Arends S, de Wolff L, van Nimwegen J, Verstappen G, Vehof J, Vissink A, Ray N, Kroese F, Bootsma H. Composite of Relevant Endpoints for Sjögren’s Syndrome (CRESS) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/composite-of-relevant-endpoints-for-sjogrens-syndrome-cress/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/composite-of-relevant-endpoints-for-sjogrens-syndrome-cress/
