Session Information
Date: Monday, November 9, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Bench to Bedside
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Mortality in patients with systemic lupus erythematosus (SLE) is significantly higher than in the general population. Treatment of SLE patients has improved, however, a majority of patients still experience symptoms like fatigue, pain and cognitive deficits even when they are clinically evaluated to have low disease activity (LDA) or being in remission. Immunosuppressants are tapered after clinical remission and patients are typically continued on hydroxychloroquine (HCQ).
Activation of the complement system is evident in most SLE patients with active disease. This is usually assessed by low C3 or C4 as a proxy for complement activation (CA). When levels normalize or stabilize, we assume CA has been controlled.
The aim of this project is to examine if SLE patients in remission or with LDA have ongoing CA. This is investigated by measuring the C3 breakdown product C3dg. In addition, we show that in vitro HCQ does not inhibit any of the CA pathways.
Methods: In 166 SLE patients and 145 age and gender-matched controls we measured the C3 breakdown product C3dg (37 kDa fragment), which is only present after activation of C3. Disease activity was assessed using SLEDAI-2K, LLDAS, PGA and remission criteria (SLEDAI-2K=0, corticosteroid-free and immunosuppressant-free, HCQ allowed). The association between CA measured by C3dg and disease activity was estimated. Further, we examined the inhibitory effect of HCQ on CA in vitro in erythrocyte cell lysis assays and by measuring deposition of activation fragments of C3 and C4 on relevant surfaces in microtiter wells, i.e. examining activation through the lectin, the classical and the alternative pathway.
Results: In this cross-sectional cohort of SLE patients the median SLEDAI-score was 4 (range 0-14), median PGA was 1 (range 0-3), 61.4 % of patients were in LLDAS (102/166) and 7.8% were in complete remission (13/166).
C3dg was significantly higher in SLE patients than in controls (p< 0.0001), whereas no significant difference was observed for C3 (p=0.67). Ongoing CA was seen in all SLE patients independently of disease activity assessment (SLEDAI-2K, PGA, LLDAS and PGA). No difference was observed between patients treated or not treated with HCQ (p=0.07). Physiological and supraphysiological concentrations of HCQ did not inhibit CA through any of the complement pathways (fig.1) or in erythrocyte cell lysis assays.
Conclusion: SLE patients have ongoing CA even when our disease assessments scores show remission or LDA.
CA is not affected by HCQ treatment in patients, which in most cases is the drug-of-choice for SLE patients in remission. This is supported by our in vitro studies demonstrating that HCQ does not inhibit CA.
Our current disease activity scores do not include the best measures of biochemically active disease like CA measures. Ongoing CA is not captured by validated clinical disease activity scores and could be an explanation for persistent symptoms reported by many patients and potentially one of the pathogenic mechanisms leading to increased mortality in SLE.
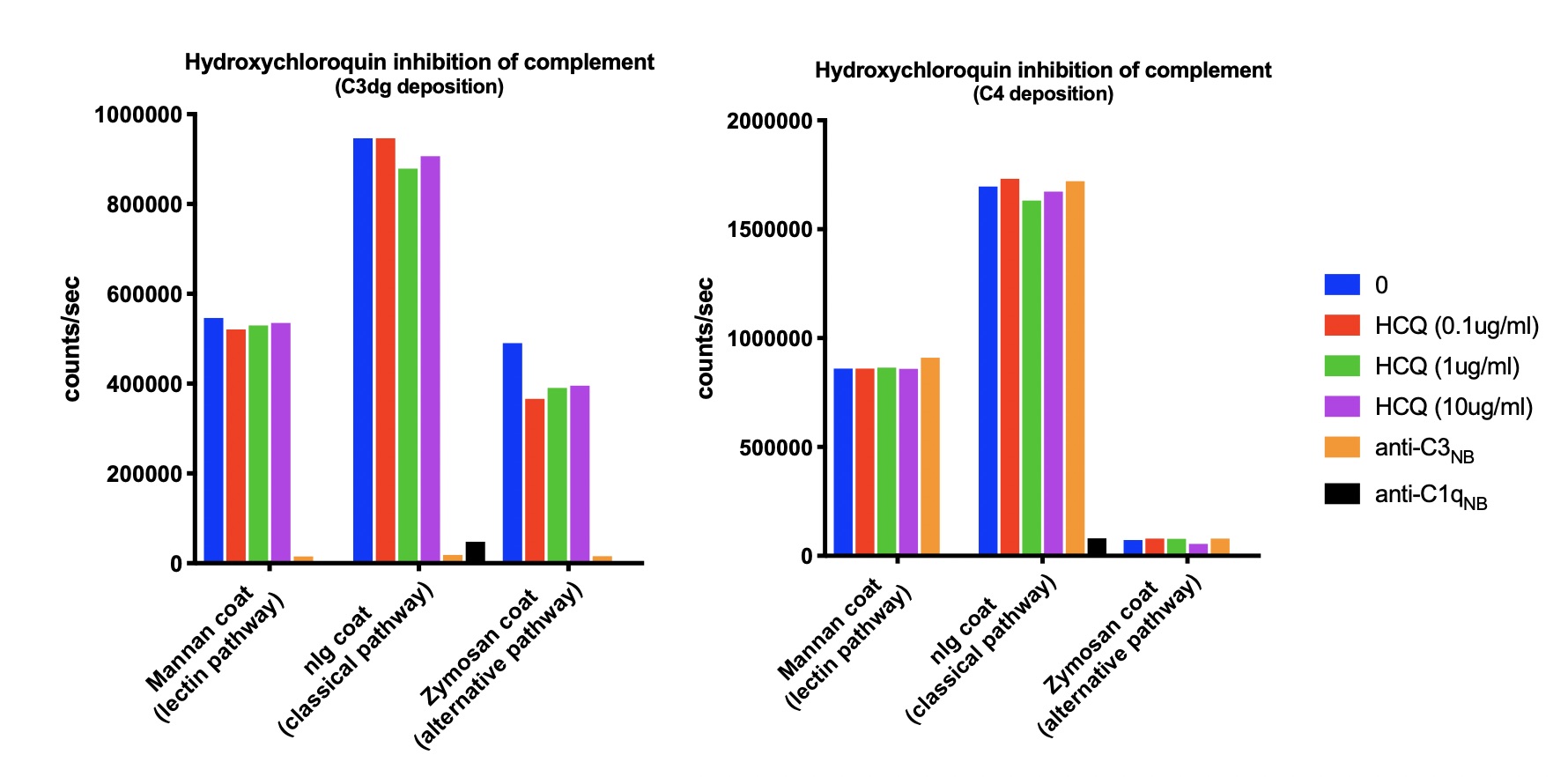 Fig.1: No inhibition of complement activation by hydroxychloroquine in vitro (HCQ).
Fig.1: No inhibition of complement activation by hydroxychloroquine in vitro (HCQ).
To cite this abstract in AMA style:
Troldborg A, Hansen A, Stengaard-Pedersen K, Thiel S. Complement Activation in Systemic Lupus Erythematosus Patients with Low Disease Activity Is Not Inhibited by Hydroxychloroquine [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/complement-activation-in-systemic-lupus-erythematosus-patients-with-low-disease-activity-is-not-inhibited-by-hydroxychloroquine/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/complement-activation-in-systemic-lupus-erythematosus-patients-with-low-disease-activity-is-not-inhibited-by-hydroxychloroquine/
