Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tocilizumab is an anti‑interleukin‑6 receptor monoclonal antibody indicated for treating rheumatoid arthritis (RA) and other inflammatory diseases. MSB11456 is a proposed biosimilar to US‑licensed tocilizumab and EU‑approved tocilizumab. It has already shown equivalent pharmacokinetic (PK), pharmacodynamic (PD) safety, tolerability, and immunogenicity profiles to these products given subcutaneously (SC) as a single dose in healthy volunteers. This is a Phase III, multicenter, randomized, double-blind, multiple fixed-dose, parallel group study aiming to compare the efficacy, safety, and immunogenicity of MSB11456 and EU – approved tocilizumab administered SC in patients with moderate-to-severe RA (NCT04512001).
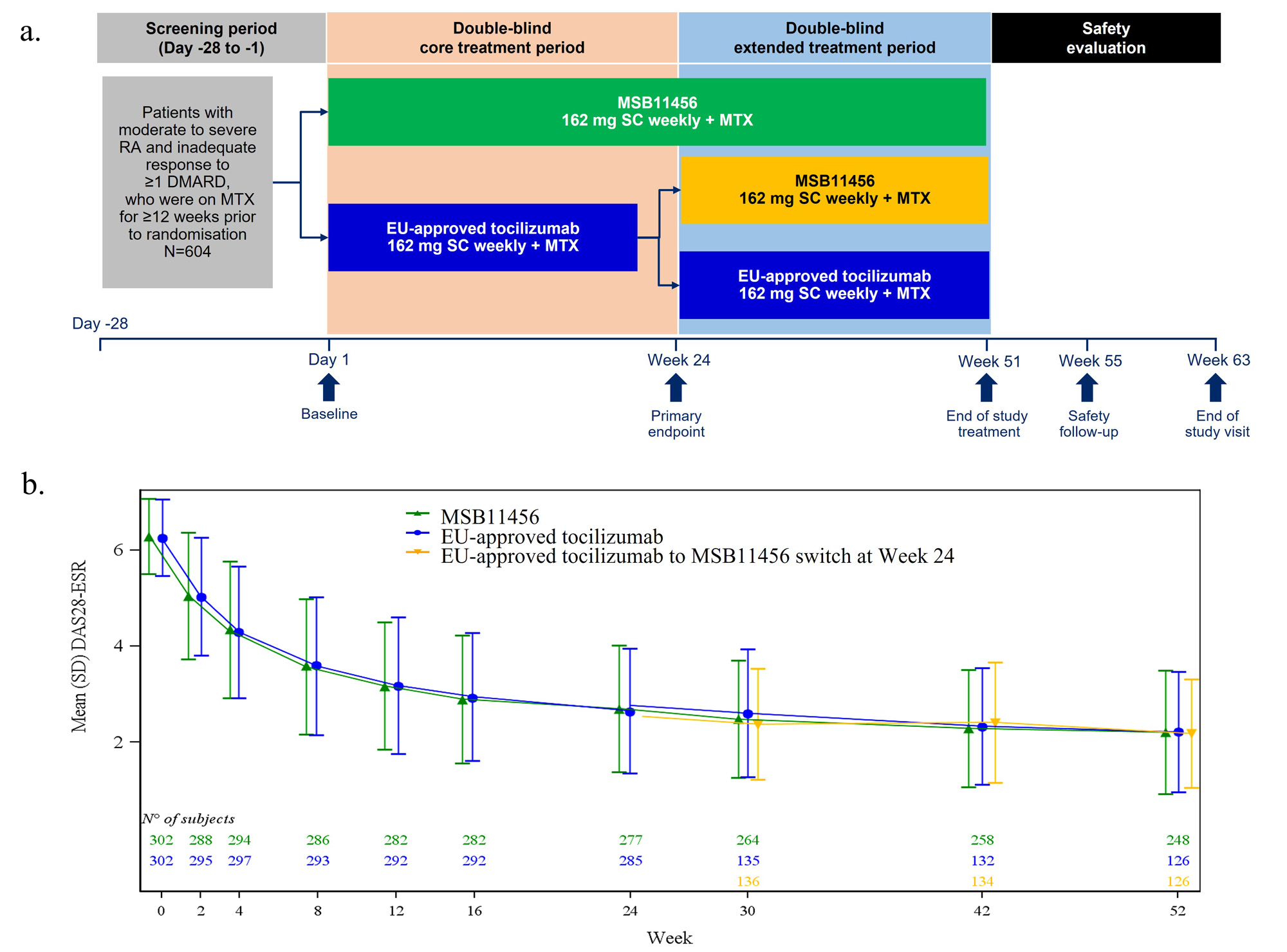
Methods: Patients were randomized to subcutaneous injections of 162 mg MSB11456 or EU – approved tocilizumab for 24 weeks (W). At W24, patients receiving EU-approved tocilizumab were re-randomized to continue their treatment or to switch to MSB11456 up to W52. Those receiving MSB11456 continued MSB11456 until W52. A safety evaluation was conducted up to W63 (Figure 1a).
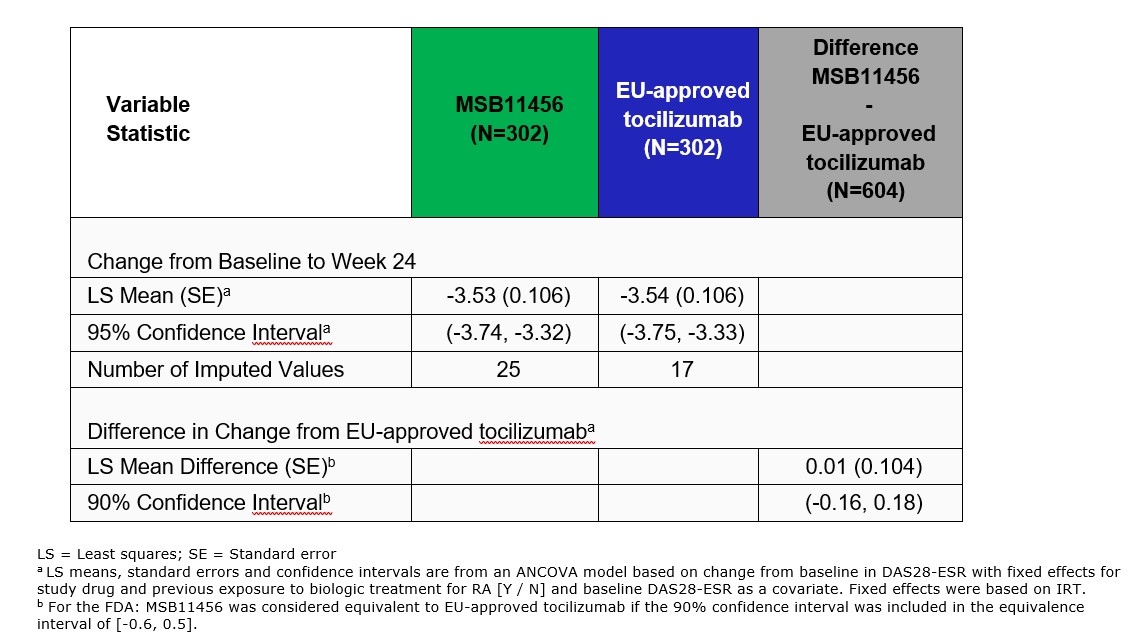
The primary efficacy endpoint – change from baseline in Disease Activity Score-28 Joint Count (DAS28)-erythrocyte sedimentation rate (ESR) at W24 – was analyzed using analysis of covariance to determine the least squares mean (LSM) difference between MSB11456 and EU-approved tocilizumab; MSB11456 was considered equivalent to EU-approved tocilizumab if the 90% confidence interval (CI) for this difference was entirely within the equivalence interval -0.6 to 0.5. Secondary endpoints were 20% improvement in American College of Rheumatology core set measures (ACR20) at W24 and DAS28-ESR at W12. Additional endpoints included ACR50/70, change in DAS28-C-reactive protein (CRP), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), evaluation of immunogenicity at various time points up to W55 and safety up to W63.
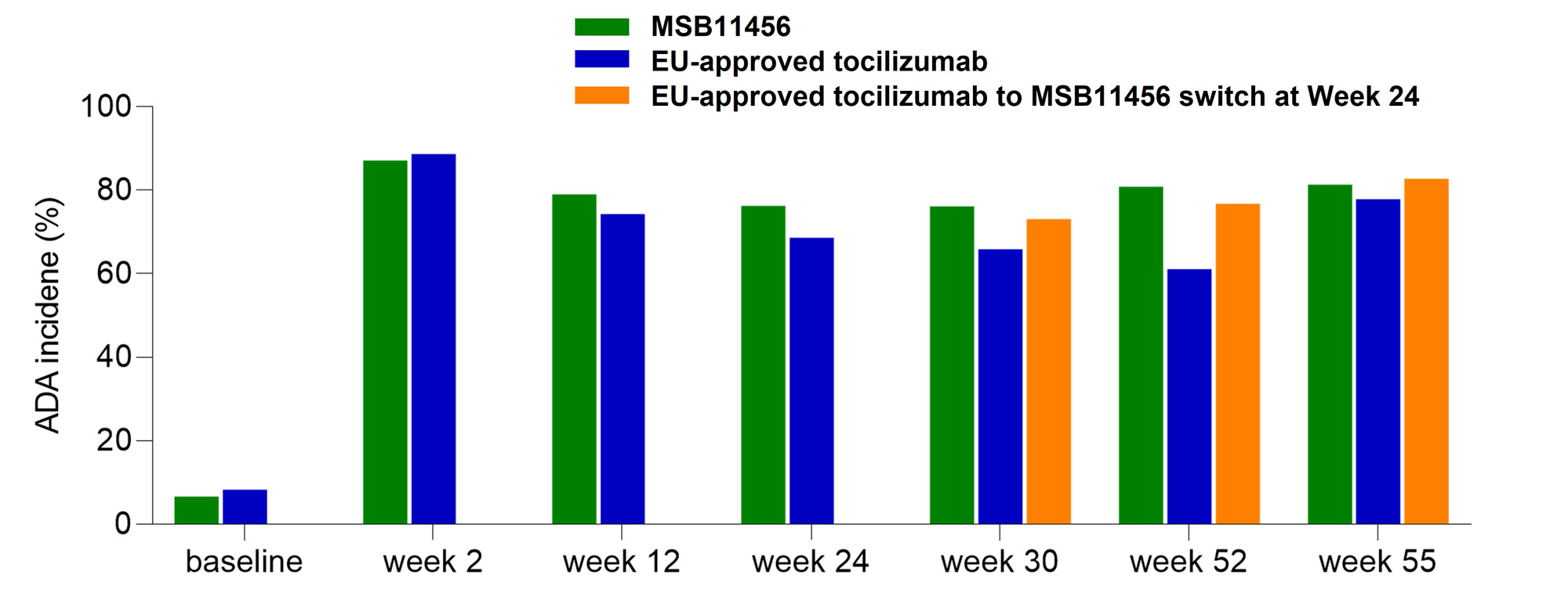
Results: Clinically relevant LSM decreases from baseline in DAS28-ESR were observed as early as W2 and up to W24 with MSB11456 and EU-approved tocilizumab (Figure 1b). As the 90% CI for the LSM difference in the change from baseline in DAS28-ESR between treatments was fully included within the predefined equivalence interval, therapeutic equivalence of MSB11456 and EU-approved tocilizumab was demonstrated (Table 1). The other efficacy endpoint analyses supported this conclusion. Treatment-emergent adverse events (TEAEs) were usually mild or moderate and occurred at similar frequency with both drugs. There were no discernible patterns in terms of the nature, frequency, or other characteristics of serious or treatment related TEAEs to suggest a difference between drugs. Anti-drug antibodies (ADA) incidence was similar among treatment arms (Figure 2). The switch from EU-approved tocilizumab to MSB11456 at W24 had no clinically relevant impact either on efficacy or safety, including immunogenicity.
Conclusion: Equivalent efficacy, and similar immunogenicity and safety profiles of MSB11456 and EU-approved tocilizumab were demonstrated in patients with moderate to severe RA
To cite this abstract in AMA style:
Zubrzycka-Sienkiewicz A, Misterska-Skora M, Socik Pojawa M, Klama K, Ullmann M, Petit-Frere C, Illes A, Baker P, Monnet J, Brzezicki J. Comparison of the Efficacy, Safety and Immunogenicity of a Proposed Biosimilar MSB11456 with Tocilizumab Reference Product in Moderate-to-severe Rheumatoid Arthritis: Results of a Randomized Double-blind Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparison-of-the-efficacy-safety-and-immunogenicity-of-a-proposed-biosimilar-msb11456-with-tocilizumab-reference-product-in-moderate-to-severe-rheumatoid-arthritis-results-of-a-randomized-double-bl/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-the-efficacy-safety-and-immunogenicity-of-a-proposed-biosimilar-msb11456-with-tocilizumab-reference-product-in-moderate-to-severe-rheumatoid-arthritis-results-of-a-randomized-double-bl/