Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Janus Kinase (JAK) inhibitors have shown long term benefit in patients with active RA with inadequate response to conventional or biologic DMARDs (1). Due to a lack of head-to-head comparison trials, the relative efficacy and safety of JAK inhibitors remains unclear. Consequently, previous network meta-analysis had assessed the relative efficacy and safety of JAK inhibitors but were restricted to studies with adalimumab (2).
The purpose of this study was to investigate the relative efficacy and safety of tofacitinib, baricitinib, filgotinib and upadacitinib in patients with active RA with inadequate response to conventional or biologic DMARDs.
Methods: Bayesian random-effects network meta-analysis was performed to combine the direct and indirect evidence from randomized controlled trials (RCTs) reporting efficacy and safety outcomes of tofacitinib + MTX, baricitinib + MTX, filgotinib + MTX and upadacitinib + MTX in patients with active RA despite treatment with conventional or biologic DMARDs.
Results: Twenty RCTs including 13.178 patients met the inclusion criteria. There were 45 pairwise comparisons including 18 direct comparisons of 10 interventions. The American College of Rheumatology 20% (ACR20) response rate was significantly higher for all the intervention groups other than placebo (figure 1). The ranking probability based on the surface under the cumulative ranking curve (SUCRA) indicated that upadacitinib 15mg + MTX (SUCRA = 0.9), tofacitinib 10mg + MTX (SUCRA = 0.8). and baricitinib 4mg + MTX (SUCRA = 0.8) had the highest probability of being the best treatment in terms of the ACR20 response rate. This was followed by upadacitinib 30mg + MTX (SUCRA = 0.6), filgotinib 200mg + MTX (SUCRA = 0.6), baricitinib 2 mg + MTX (SUCRA 0.4), tofacitinib 5mg + MTX (SUCRA = 0.3), filgotinib 100mg + MTX (SUCRA = 0.3), adalimumab + MTX (SUCRA = 0.2) and placebo + MTX (SUCRA = 0). The SUCRAs for the ACR50 indicated that upadacitinib 30mg + MTX (SUCRA = 0.9) and upadacitinib 15mg (SUCRA =0.8) had the highest probability of being the best treatment. Safety based on number of severe adverse events did not differ significantly among the ten interventions within 12 weeks suggesting comparable safety among the different regimens and the placebo. However, it was observed an increased risk of herpes zoster infection in the group of tofacitinib 10mg + MTX (odds ratio (OR) 8.27, 95% credible interval (CrI) 1.56 – 43.7) and baricitinib 4mg + MTX (OR 2.36, 95% CrI 1.1 – 5.1) in comparison with placebo group (Table 1).
Conclusion: In patients with active RA with inadequate response to conventional or biologic DMARDs the JAK inhibitors are an effective and safe alternate therapy. The two with the best relative efficacy were upadacitinib 15mg/30mg and baricitinib 4 mg. In terms of serious infectious events, there was an increase of herpes zoster infections with tofacitinib 10mg and baricitinib 4mg.
References
- Smolen JS, et al. Annals of the Rheumatic Diseases. 2020 Jan 22. doi: 10.1136/annrheumdis-2019-216655.
- Lee YH, et al. Z Rheumatol. 2020 Feb 13. doi: 10.1007/s00393-020-00750-1.
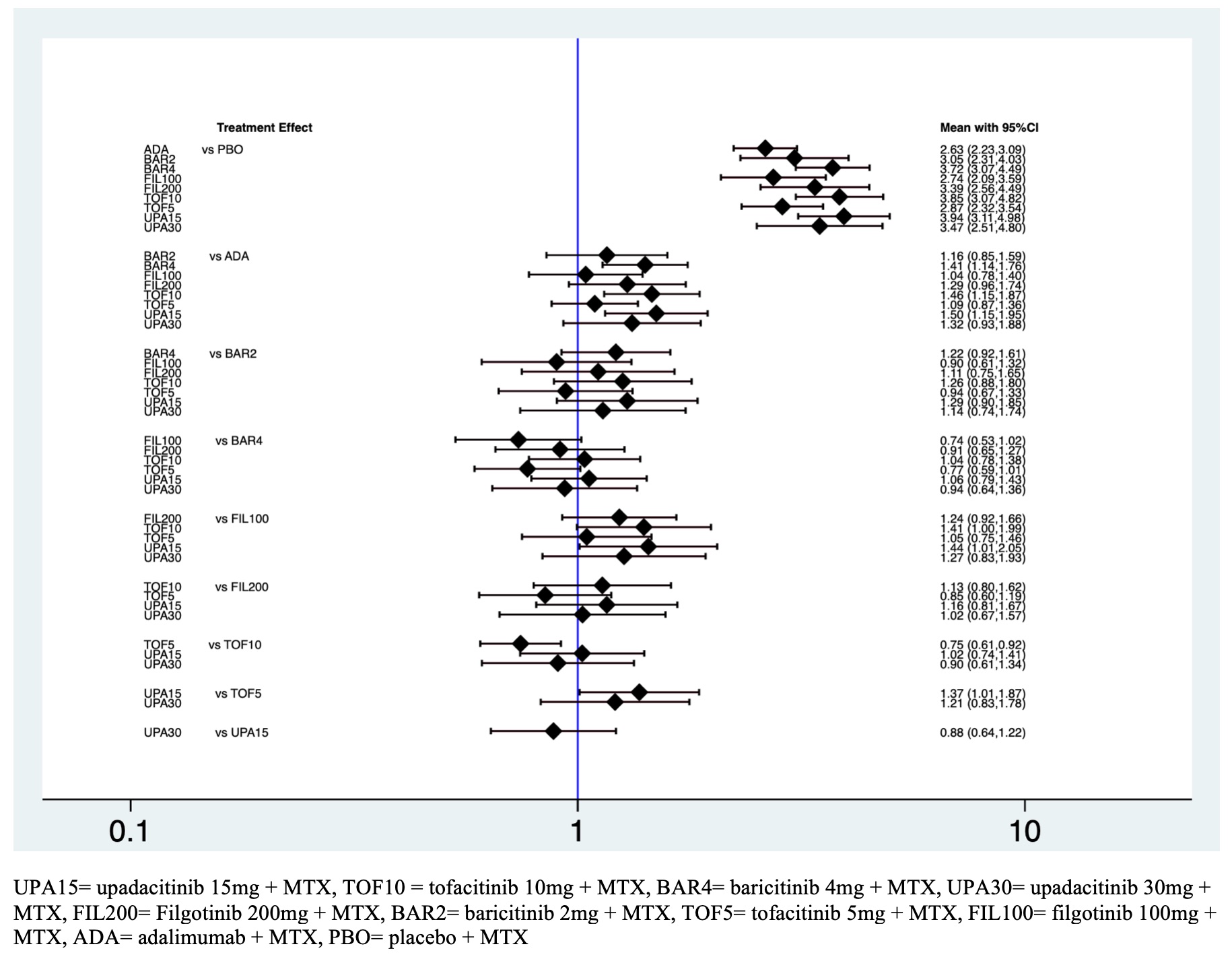 Figure 1. Predictive interval plot for active RA network for the ten-intervention analysis for ACR20.
Figure 1. Predictive interval plot for active RA network for the ten-intervention analysis for ACR20.
 Table 1. Network league of estimated effects of ACR20 and herpes zoster infection.
Table 1. Network league of estimated effects of ACR20 and herpes zoster infection.
To cite this abstract in AMA style:
Castro A, Diaz J, Quiceno G. Comparison of the Efficacy and Safety of Janus Kinase Inhibitors and DMARDs in Patients with Active Rheumatoid Arthritis: A Bayesian Network Meta-Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparison-of-the-efficacy-and-safety-of-janus-kinase-inhibitors-and-dmards-in-patients-with-active-rheumatoid-arthritis-a-bayesian-network-meta-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-the-efficacy-and-safety-of-janus-kinase-inhibitors-and-dmards-in-patients-with-active-rheumatoid-arthritis-a-bayesian-network-meta-analysis/
