Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
2 Novartis Ireland Ltd, Dublin, Ireland
3 Novartis Healthcare Pvt. Ltd., Hyderabad, India
4 Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA
5 Novartis Pharma AG, Basel, Switzerland
Background/Purpose: The objective of this analysis was to estimate and compare the long-term cost per responder based on the American College of Rheumatology outcomes (ACR 20/50/70) following 48 weeks of psoriatic arthritis (PsA) treatment with the anti-IL-17A antibody Secukinumab relative to the anti-TNF antibody Adalimumab.
Methods: The cost per responder for each treatment, namely Secukinumab and Adalimumab was estimated by dividing the drug acquisition cost for the course of treatment with its response rate. Drug costs were estimated on the basis of the official US drug acquisition costs and the number of doses required for 48 weeks. The long-term response rates were estimated using a matching-adjusted indirect comparison (MAIC) technique based on the data from FUTURE 2 and ADEPT clinical trials of Secukinumab and Adalimumab respectively. MAIC analysis matched the age, weight, race and gender distribution, PASI score, HAQ-DI score, PsA duration (years), swollen joint count, CRP , proportions of patients using methotrexate, with psoriasis ≥3% body surface area, presence of dactylitis, presence of enthesitis and TNF-naive at the baseline. A sensitivity analysis was also conducted by varying the choice of baseline characteristics (includes all variables except PsA duration (years), swollen joint count, and CRP) used in MAIC analysis.
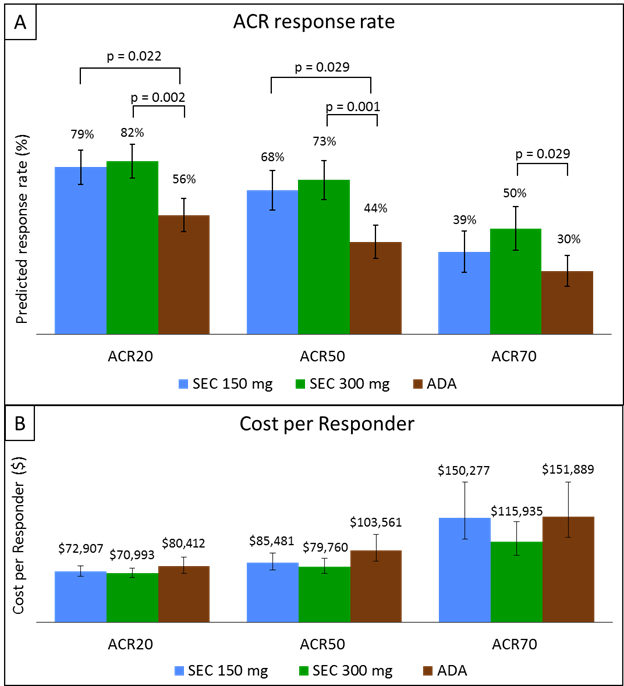
Results: The effective sample sizes after matching the baseline characteristics were 15, 25 for Secukinumab 150 mg, Secukinumab 300 mg respectively, while the sample size for Adalimumab was 151. MAIC analysis showed ACR (20/50/70) response rates were significantly higher for Secukinumab 150mg and 300mg compared to Adalimumab at 48 weeks. ACR 20 response rates were 79%, 82% and 56% ACR 50 response rates were 68%, 73% and 44%, whereas the ACR 70 response rates were 39%, 50% and 30% for Secukinumab 150mg, Secukinumab 300 mg and Adalimumab respectively (Figure 1A). Sensitivity analysis also showed higher response rates for Secukinumab 150mg and 300mg compared to Adalimumab. Among PsA patients, cost per ACR20 responder were $72,906, $70,993 and $80,412 cost per ACR50 responder were $85,480, $79,760 and $103,561, whereas costs per ACR70 responder were $150,276, $115,934 and $151,890 for Secukinumab 150mg, Secukinumab 300mg and Adalimumab respectively (Figure 1B). Sensitivity analysis also produced similar results.
Conclusion: ACR (20/50/70) response rates were significantly higher for Secukinumab 150mg and 300mg compared to Adalimumab at 48 weeks. The long term cost per responder for all ACR outcomes at 48 weeks were consistently lower for secukinumab (150,300mg) vs. adalimumab. These findings indicate that Secukinumab represents a cost-efficient treatment choice for PsA patients in the US.
Figure 1: Predicted response rate (A) and cost per responder (B) analysis of ACR (20/50/70) for Secukinumab vs Adalimumab for the treatment of psoriatic arthritis at 48 weeks in principal analysis
To cite this abstract in AMA style:
Greenberg JD, Nikoglou E, Gunda P, Palmer J, Jugl S. Comparison of Secukinumab Vs Adalimumab in a Cost per Responder Analysis Based on a Matching-Adjusted Indirect Comparison of Efficacy Data for the Treatment of Psoriatic Arthritis at 48 Weeks from the US Perspective [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/comparison-of-secukinumab-vs-adalimumab-in-a-cost-per-responder-analysis-based-on-a-matching-adjusted-indirect-comparison-of-efficacy-data-for-the-treatment-of-psoriatic-arthritis-at-48-weeks-from-the/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-secukinumab-vs-adalimumab-in-a-cost-per-responder-analysis-based-on-a-matching-adjusted-indirect-comparison-of-efficacy-data-for-the-treatment-of-psoriatic-arthritis-at-48-weeks-from-the/