Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The 2022 ESC/ERS Guidelines recommend comprehensive risk stratification at diagnosis of pulmonary arterial hypertension (PAH) to guide optimized management.1Several risk stratification tools have been developed with data derived mainly from patients with idiopathic PAH. However, patients with systemic sclerosis (SSc)-associated PAH have a worse prognosis. We aimed to assess the performance of current risk stratification tools to predict mortality in SSc-PAH by adding variables specific to SSc.
Methods: We included SSc patients from the EUSTAR database who were diagnosed with PAH by right heart catheterization (RHC) between 2001-2021 (Project Number: CP122). PAH was defined as a mean pulmonary arterial pressure >20 mmHg, pulmonary artery wedge pressure ≤15 mmHg and pulmonary vascular resistance >2 WU. We excluded patients with previous PAH-specific treatment and meaningful interstitial lung disease (ILD), defined as an extent of ILD >20% on HRCT or FVC < 70% in patients with missing quantification. We applied four different approaches:
(I) 2022 ESC/ERS 3-strata: Three risk groups based on the mean of up to 17 risk parameters from the guidelines graded 1-3 representing low-high risk
(II) 2022 ESC/ERS 4-strata: Equals no. (I), but divides the intermediate-risk group into two groups
(III) COMPERA 2.0: Four risk groups based on the mean of WHO-functional class (FC) and/or six-minute walk distance (6MWD) and NT-proBNP graded 1-4 representing low-high risk.2
(IV) REVEAL Lite 2:Three risk groups based on six weighted variables (WHO-FC, systolic blood pressure, heart rate, 6MWD, NT-pro-BNP, and eGFR)3
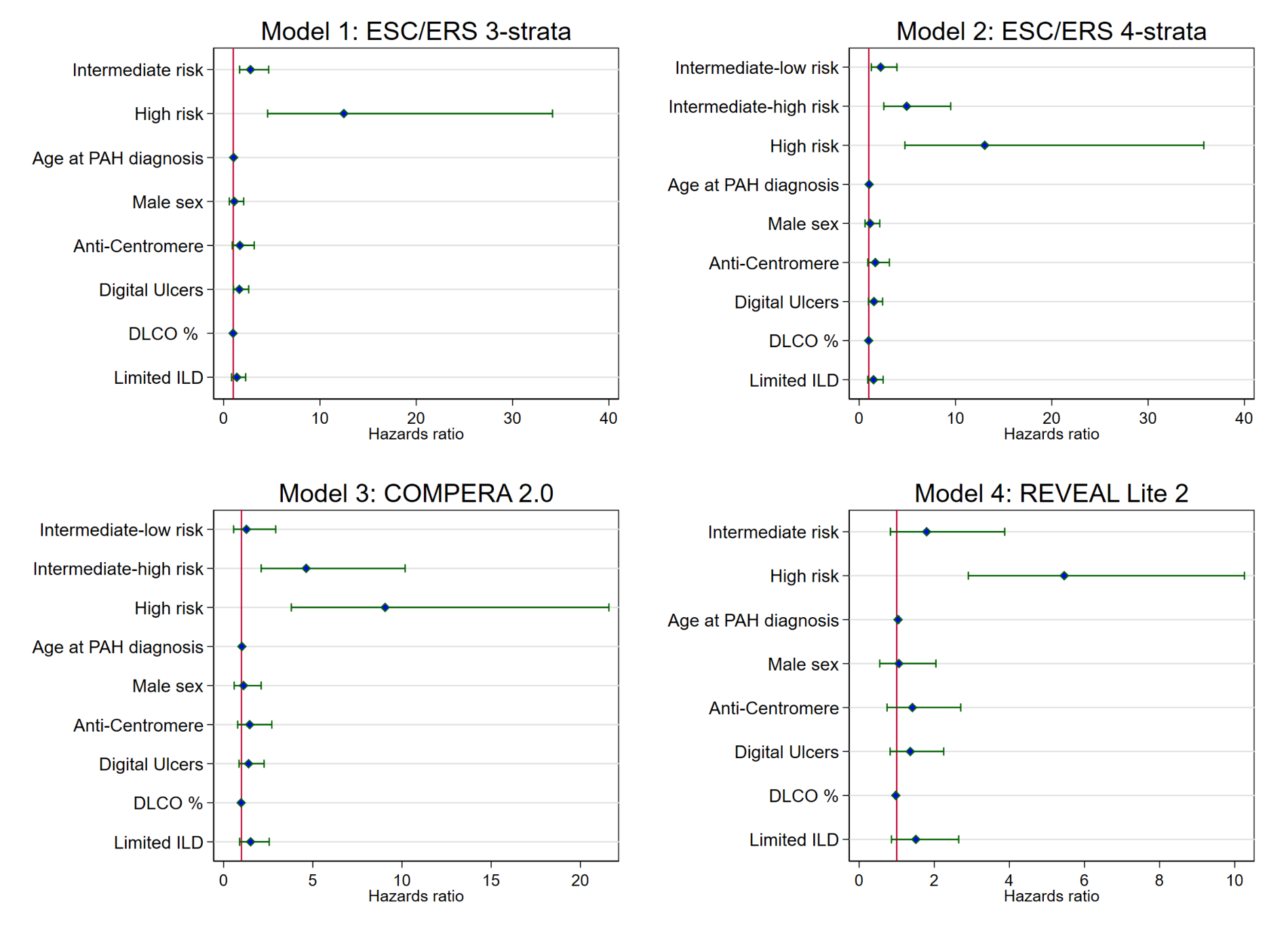
We performed Cox regression adjusted for general and SSc-specific factors associated with worse outcome based on expert opinion and published literature (age, male sex, anti-centromere antibodies, digital ulcers, DLCO and limited ILD). Harrell’s C-index and ROC analysis with area under the curve (AUC) were applied to compare the performance and discriminating ability of the models with >0.7 defined as acceptable.
Results: Of 890 patients who had RHC, 367 were eligible. Among these, 87% were females, mean age was 66 years, and 83% had limited cutaneous SSc.
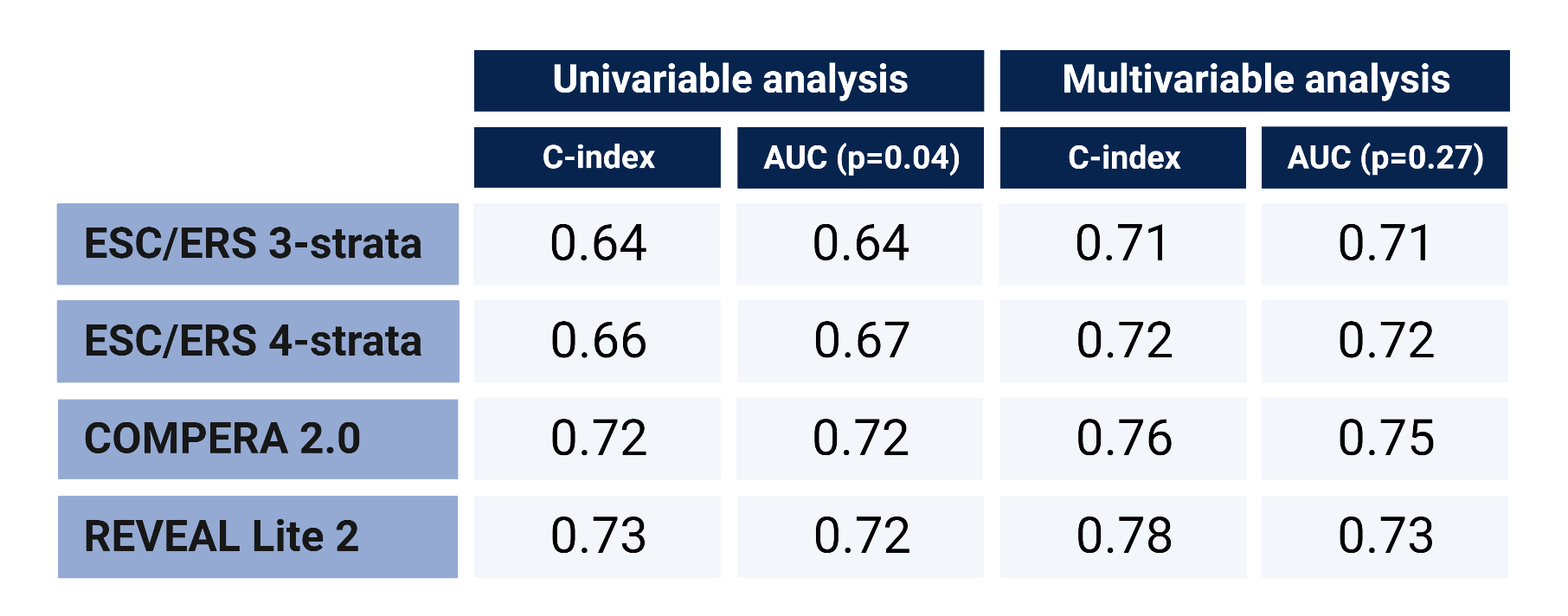
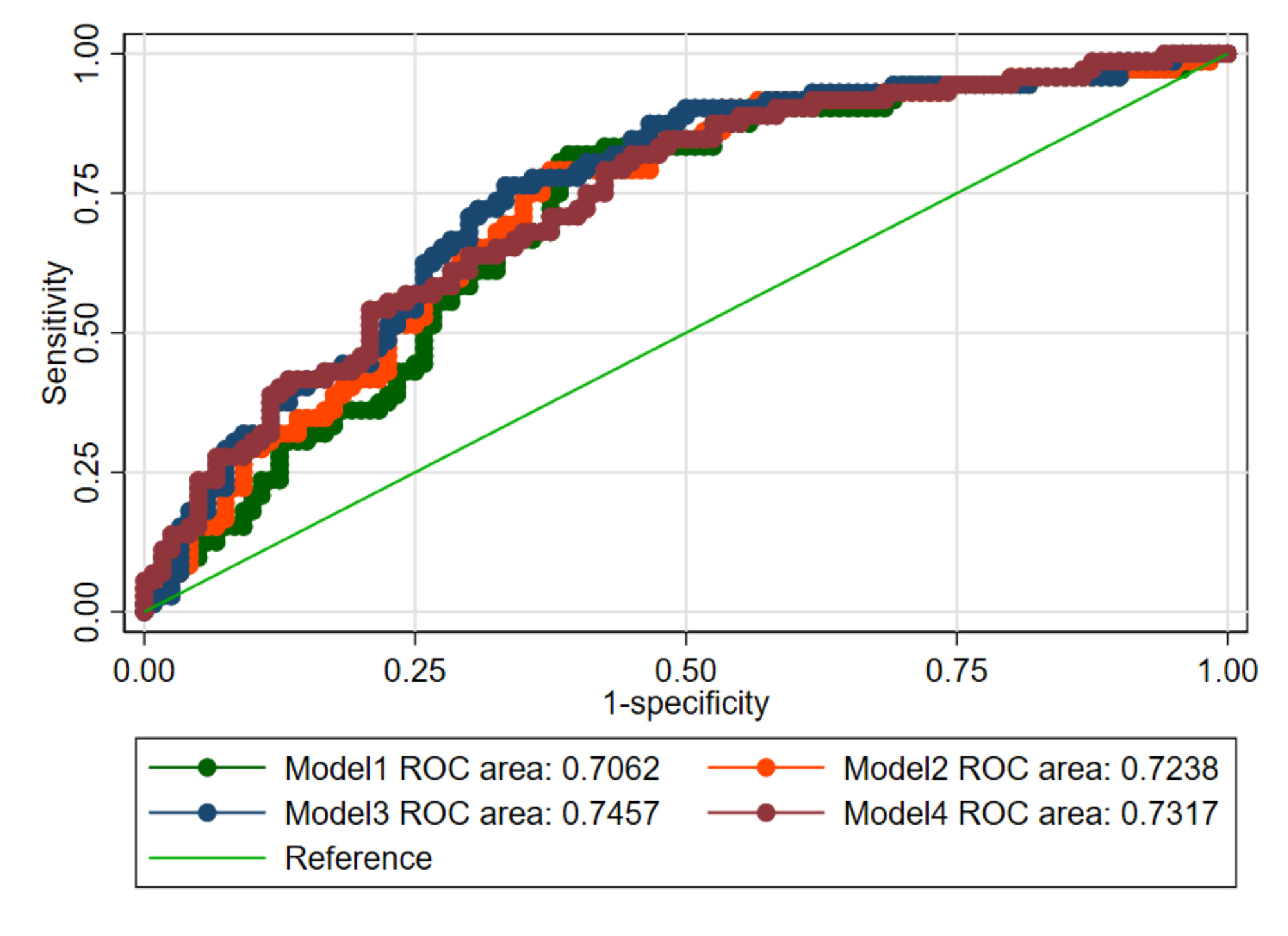
In univariable analysis, only COMPERA 2.0 and REVEAL Lite 2 had acceptable predictive value with a C-index >0.7 (table). Adjusted for general and SSc-specific variables, all models were acceptable (table, fig. 1).Numerically, COMPERA 2.0 and REVEAL Lite 2 were the most accurate to predict mortality (table, fig. 1). Hazard ratios increased with higher risk scores (fig. 2). However, COMPERA 2.0 and REVEAL Lite 2 did not significantly discriminate the lower risk groups (fig. 2).
Conclusion: In SSc-PAH, we suggest that risk stratification at time of PAH diagnosis should take general and SSc-specific variables into account. The COMPERA 2.0 and REVEAL Lite 2 risk stratification models perform best when used as stand-alone tools. Neither requires invasive measurements, making them easily applicable in clinical practice. Importantly, these models identify patients in the high-risk group where aggressive upfront treatment is recommended.
References:
1Humbert, Eur Heart J, 2022
2Hoeper, Eur Respir J, 2022
3Benza, Chest, 2021
To cite this abstract in AMA style:
Jenssen Bjørkekjær H, Bruni C, Brunborg C, Carreira P, Airò P, Simeon-Aznar C, Truchetet M, Giollo A, Balbir-Gurman A, Martin M, Denton C, Gabrielli A, Fretheim H, Barua I, Bitter H, Midtvedt O, Garen T, Broch K, Andreassen A, Tanaka Y, Riemekasten G, Müller-Ladner U, Matucci Cerinic m, Castellvi I, Siegert E, Hachulla E, Distler O, Hoffmann-Vold A. Comparison of Four Risk Stratification Models for Prediction of Mortality in Systemic Sclerosis-associated Pulmonary Arterial Hypertension in the EUSTAR Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparison-of-four-risk-stratification-models-for-prediction-of-mortality-in-systemic-sclerosis-associated-pulmonary-arterial-hypertension-in-the-eustar-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-four-risk-stratification-models-for-prediction-of-mortality-in-systemic-sclerosis-associated-pulmonary-arterial-hypertension-in-the-eustar-cohort/