Session Information
Date: Sunday, November 17, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Ultrasound is frequently used in rheumatology practice to assist with the diagnosis of rheumatic and musculoskeletal diseases. In patients with gout, it allows visualization of monosodium urate (MSU) crystal deposition as double contour, aggregates and tophus. Use of handheld portable ultrasound is increasing and would improve access for people with rheumatic disease when conventional, cart-based ultrasound is unavailable. This study compared handheld and cart-based ultrasound for the assessment of gout lesions in people with established gout.
Methods: The lower limbs of 21 participants with gout (mean duration of gout 12 years, 90% on urate-lowering therapy, mean serum urate 0.34 mmol/L) were independently scanned at six sites (1st and 2nd metatarsophalangeal joints, knee, patellar ligament, Achilles tendon, and peroneal tendons) using cart-based (LOGIQ P9) and handheld (Vscan AirTM) ultrasound by two rheumatologists. One rheumatologist was randomized to scan the right or left leg first with the cart-based or handheld ultrasound. The other rheumatologist scanned the legs in the opposite order with the imaging devices reversed. Images were saved and blinded images assessed by two rheumatologists experienced in gout ultrasound for the presence of double contour, tophus, erosion and aggregates using OMERACT definitions.
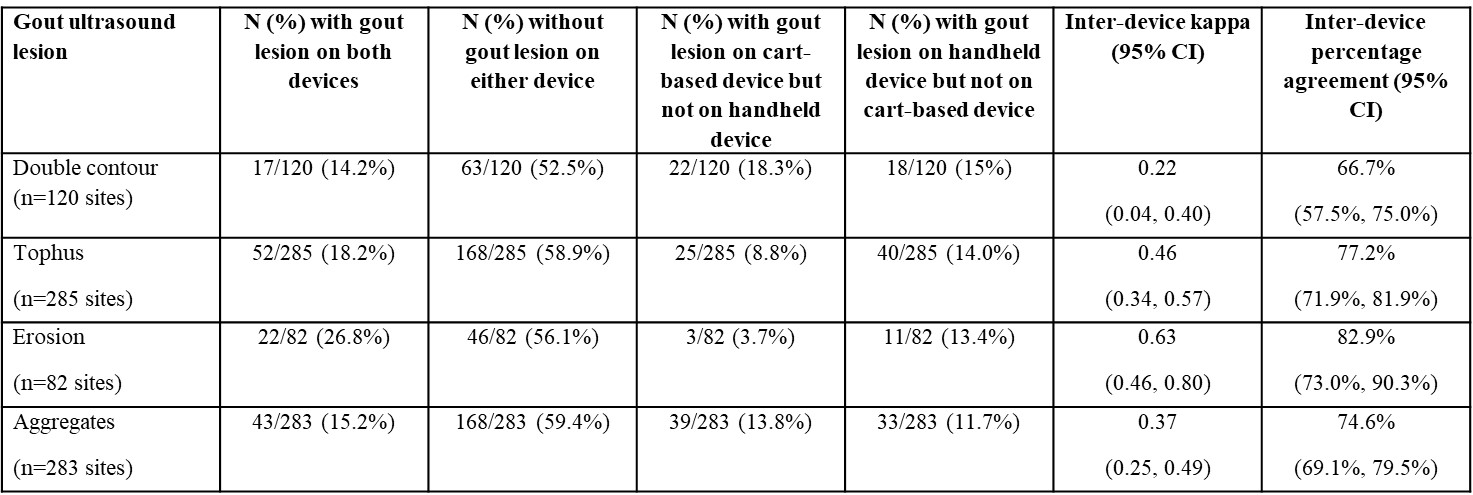
Results: A total of 41 limbs in 21 participants were scanned. On handheld ultrasound, 19/21 (90%) of participants had at least one site with double contour, tophus and erosions, and 21/21 (100%) had at least one site with aggregates. There were similar findings using cart-based ultrasound. However, site-level inter-device analysis showed only fair-good agreement: kappa (percentage agreement) for double contour 0.22 (67%), tophus 0.46 (77%), erosion 0.63 (83%) and aggregates 0.37 (75%) (Table 1). There were more aggregates detected by cart-based ultrasound in joints and more tophi detected by handheld ultrasound in ligaments and tendons (data not shown).
Conclusion: Handheld ultrasound can detect gout lesions in people with established gout and has potential value in assisting with identification of gout lesions in patients who do not have access to cart-based ultrasound. However, concordance between cart-based and handheld ultrasound in detection of some gout lesions is low, particularly double contour and aggregates.
To cite this abstract in AMA style:
Murdoch R, Terslev L, Martin J, Mihov B, Gamble G, Torp-Pedersen S, Horne A, Dalbeth N. Comparison of a Handheld Ultrasound Device with Cart-Based Ultrasound for the Assessment of Gout Lesions in People with Established Gout [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparison-of-a-handheld-ultrasound-device-with-cart-based-ultrasound-for-the-assessment-of-gout-lesions-in-people-with-established-gout/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-a-handheld-ultrasound-device-with-cart-based-ultrasound-for-the-assessment-of-gout-lesions-in-people-with-established-gout/