Session Information
Session Type: Abstract Session
Session Time: 10:30AM-11:30AM
Background/Purpose: Immune checkpoint inhibitor associated arthritis (ICI-A) affects ∼4% of ICI-treated cancer patients and often persists. Given its long duration, it is important to identify effective treatments that do not interfere with cancer responses. However, there are no studies comparing the safety or effectiveness of disease modifying anti-rheumatic drugs (DMARDs) for this condition.
Methods: This was a retrospective multicenter observational study. Inclusion criteria were 1. ICI-A or ICI-associated PMR with ≥ 1 inflamed peripheral joint and 2. Treatment with a TNF or IL6R inhibitor (TNFi or IL6Ri), and/or methotrexate (MTX). Patients with a preexisting autoimmune disease were excluded. The primary outcome was time to cancer progression from time of ICI initiation. The secondary outcome was time to arthritis control, defined as grade 1 arthritis (mild pain, not interfering with function) and prednisone ≤ 10 mg after DMARD initiation. Descriptive statistics and comparisons were performed using Fisher’s exact test, T-Tests, Wilcoxon rank sum tests and ANOVA as appropriate. Cox proportional hazard models were constructed to identify factors associated with cancer progression and arthritis control. Variables included in the models were DMARD (exposure of interest), ICI and cancer type, age, sex, and time from ICI to DMARD initiation.
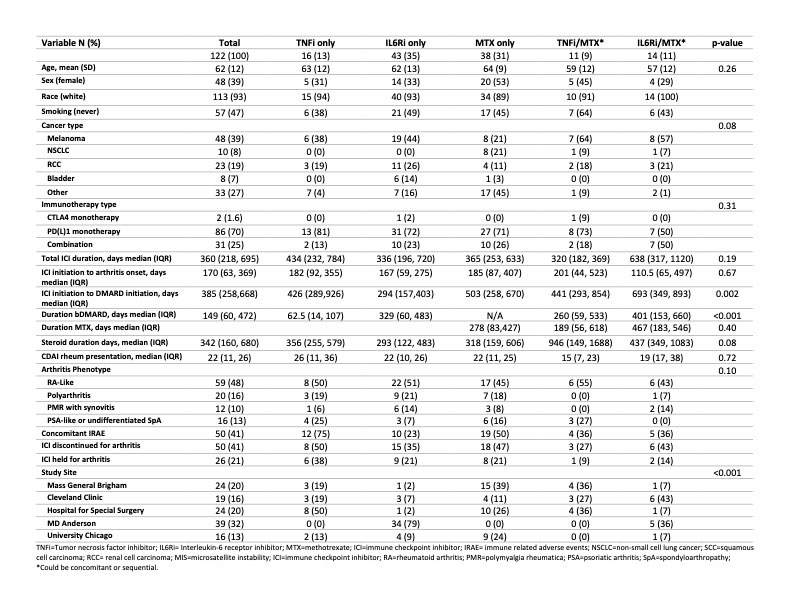
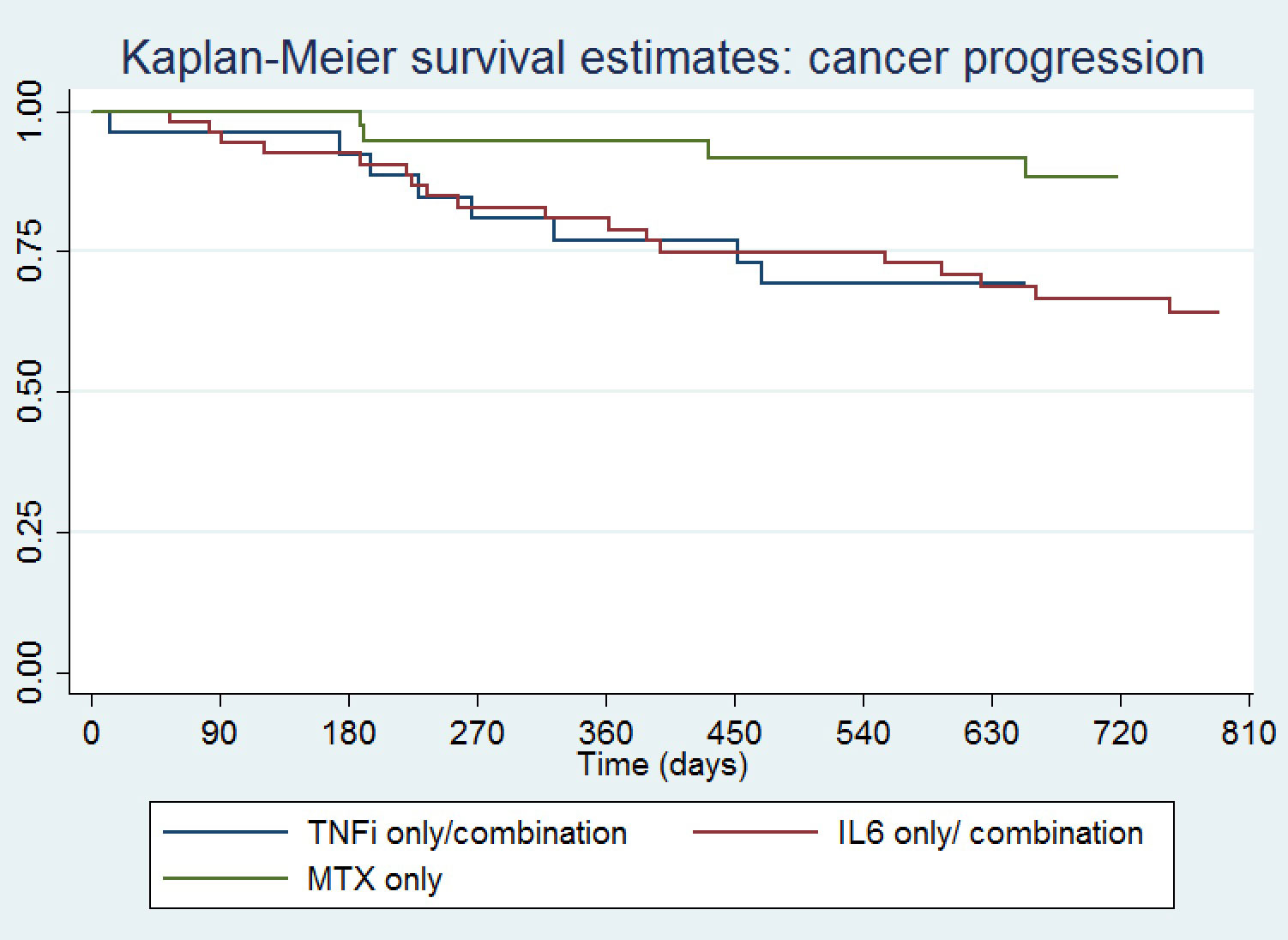
Results: One hundred twenty-two patients were included, mean (SD) age 62 (12) years, 39% female, 39% with melanoma, 8% non-small cell lung cancer, 19% renal cell carcinoma and 7% bladder cancer (Table 1). Seventy percent received PD(L)1 monotherapy. Median (IQR) time from ICI initiation to ICI-A onset was 170 (63,369) days, and from ICI-A onset to initiation of a DMARD 385 (258,668) days. Time from ICI initiation to DMARD start was shortest for IL6Ri-only treated patients. Median CDAI at rheumatology presentation was 22 (11,26) and 41% of the patients had other concomitant immune related adverse events. ICI were held for ICI-A in 21% of patients and discontinued in 41%. The DMARD used was a TNFi in 13%, IL6Ri 35%, MTX 31%, TNFi/MTX 9% and IL6Ri/MTX 11%. Total duration of DMARD treatment was shortest for TNFi-only treated patients. A Kaplan-Meier curve showing time to cancer progression by DMARD treatment is shown in Figure 1, and time to arthritis control in Figure 2. Compared to patients treated with a TNFi ± MTX, patients treated with IL6Ri ± MTX had a similar time to cancer progression (HR 0.74; 95%CI 0.32-1.72, P=0.49) and to arthritis control (HR 0.84; 95%CI 0.46-1.50, P=0.55). Patients treated with MTX alone had a trend toward a longer time to cancer progression (HR 0.39; 95%CI 0.13-0.1.19, P=0.10) and a longer time to arthritis control (HR 0.37; 95%CI 0.19-0.72, P=0.004) than patients treated with a TNFi ± MTX.
Conclusion: This retrospective cohort study suggests that ICI-A patients treated with either a TNFi ± MTX or an IL6Ri ± MTX have a shorter time to cancer progression but faster arthritis control than patients treated with MTX alone. A prospective randomized controlled trial is needed to verify these findings and to identify the optimal approach to managing patients with high grade ICI-A.
To cite this abstract in AMA style:
Bass A, Abdel-Wahab N, Reid P, Sparks J, Calabrese C, Jannat-Khah, DrPH, MSPH D, Rajesh D, Ghosh N, Mushtaq K, Al Haj F, Falohun A, Gedmintas L, MacFarlane L, Arabelovic S, Diab A, Shah A, Bingham III C, Chan K, C. Cappelli L. Comparing the Safety and Effectiveness of Methotrexate, TNF and IL6 Inhibitors for the Treatment of Checkpoint Inhibitor Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/comparing-the-safety-and-effectiveness-of-methotrexate-tnf-and-il6-inhibitors-for-the-treatment-of-checkpoint-inhibitor-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-the-safety-and-effectiveness-of-methotrexate-tnf-and-il6-inhibitors-for-the-treatment-of-checkpoint-inhibitor-arthritis/