Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In the context of the recent update of the ASAS core outcomes set (COS), the preferred comparative validity of the measurement instruments to assess the domains ‘Pain’ and ‘Stiffness’ has been questioned as for each domain responsiveness of instruments was comparable. Group discrimination across various external constructs can help provide useful insights and represents unmet need.
Our objective was to compare the group discriminatory capacity, as part of the construct validity, of three instruments to assess pain and three questions of morning stiffness.
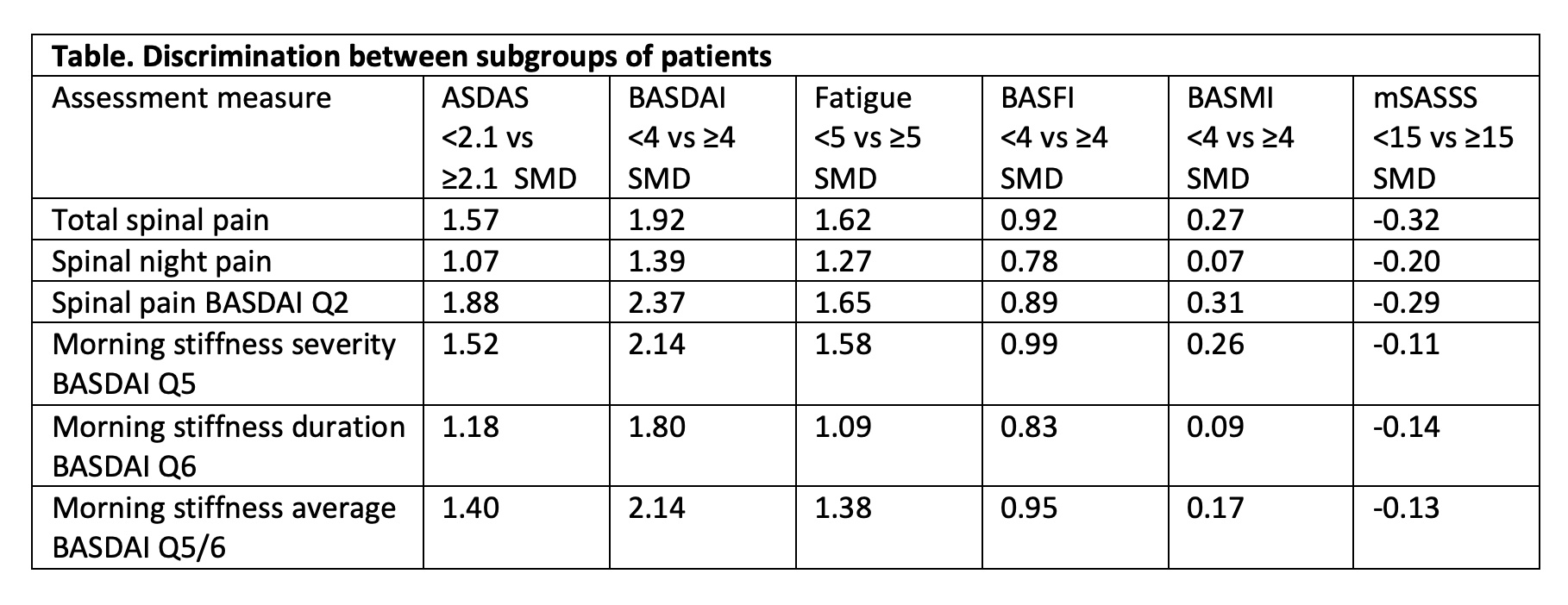
Methods: Data from the 8-year visit of patients with axSpA from the multinational OASIS cohort was assessed. The available instruments for pain assessment were: i) total spinal pain, ii) spinal pain at night, iii) spinal pain from BASDAI Q2 (addressing neck, back or hip pain); and for morning stiffness: i) severity of morning stiffness (BASDAI Q5), ii) duration of morning stiffness (BASDAI Q6), iii) the combined score between severity and duration of morning stiffness (average BASDAI Q5/6). Data from 8 year-visit were used as the first time-point where all the instruments were obtained on a 0–10 numeric rating scale (as currently used). The discriminatory capacity was assessed through the standardised mean difference (SMD) that is calculated as the difference of the group means divided by the pooled SD of the group means, with a higher value reflecting a higher discriminatory capacity. The external constructs used to compare the ability to discriminate between subgroups of patients were: ASDAS, BASDAI (dichotomized into inactive/active disease), PGA, PhGA, fatigue, BASFI, BASMI and mSASSS (dichotomized by the median).
Results: 98 patients were included: 71% males, mean age 54 (SD 11), with a mean symptom duration of 31 (11) years. The mean scores for pain were 3.7 (2.3), 2.9 (2.3) and 4.6 (2.6) for spinal pain, spinal pain at night and BASDAI Q2, respectively. The mean scores of morning stiffness were 3.7 (2.6), 3.3 (3.1) and 3.5 (2.7) for BASDAI Q5, Q6 and Q5/6, respectively. Spinal pain by BASDAI Q2 and total spinal pain had higher SMDs compared to spinal night pain across all group comparisons, with spinal pain BASDAI Q2 performing mostly slightly better (Table). Regarding morning stiffness, the severity question (BASDAI Q5) had consistently higher SMDs across all the clinical external constructs, while duration of morning stiffness (BASDAI Q6) performed worse.
Conclusion: Spinal pain from BASDAI Q2 and severity of morning stiffness (BASDAI Q5) are, respectively, the pain and morning stiffness instruments that best discriminate subgroups of patients classified according to disease activity, functional ability, fatigue or spinal mobility. The recommended ASAS COS pain instrument spinal pain BASDAI Q2, was confirmed to discriminate best. In the case of stiffness, the ASAS COS stiffness measure (BASDAI Q5/6) performed well although slightly less than the severity of morning stiffness (BASDAI Q5).
To cite this abstract in AMA style:
Capelusnik D, Nikiphorou E, Boonen A, van der Heijde D, Landewé R, van Tubergen A, Ramiro S. Comparing the Construct Validity Among Measures of Pain and Stiffness in Patients with Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparing-the-construct-validity-among-measures-of-pain-and-stiffness-in-patients-with-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-the-construct-validity-among-measures-of-pain-and-stiffness-in-patients-with-axial-spondyloarthritis/