Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The Disease Activity index for PSoriatic Arthritis based on 66/68 joints (DAPSA66/68) and the Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP) are two widely used disease activity scores to monitor patients with psoriatic arthritis (PsA). A modified DAPSA based on 28 joints (DAPSA28) has been proposed and validated in patients with PsA. We aimed to identify and compare predictors of clinical remission according to DAPSA28 (≤4), DAPSA66/68 (≤4) and DAS28-CRP (< 2.6) in a broader population of European patients with PsA initiating a first tumor necrosis factor inhibitor (TNFi).
Methods: Prospectively collected real-world data from PsA patients initiating a first TNFi between 2009 and 2018 from nine countries participating in the European Spondyloarthritis (EuroSpA) Research Collaboration Network were pooled. Patients with complete data on DAPSA28, DAPSA66/68 as well as DAS28-CRP at 6-month follow-up (from 90 to 270 days after treatment start date) constituted the study cohort. Logistic regression analyses on multiple imputed baseline data (20 imputed datasets) were used to identify predictors of DAPSA28, DAPSA66/68 and DAS28-CRP remission at 6 months. Fifteen baseline (from 30 days before to 30 days after treatment start date) demographic and clinical characteristics were included as potential predictors. For each remission outcome, variable selection was performed separately in each imputed dataset. Predictors that appeared in at least half of the datasets were included in the final model.
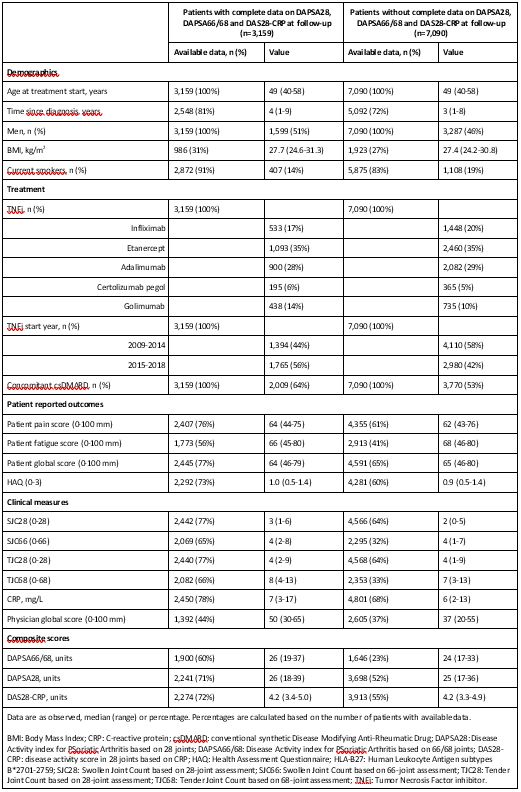
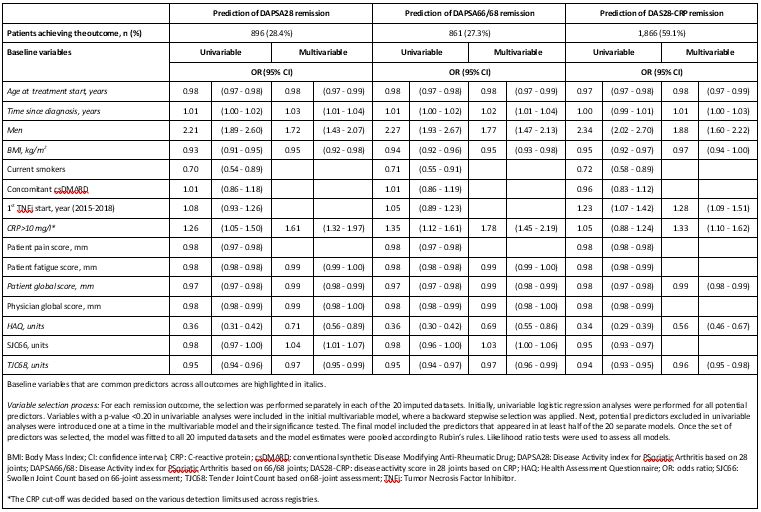
Results: Of 10,249 PsA patients, 3,159 (30.8%) had complete assessments of DAPSA28, DAPSA66/68 and DAS28-CRP at 6 months and were included in the analyses. Baseline characteristics of included patients were similar to those not included (Table 1). Among included patients, 896 (28.4%), 861 (27.3%), and 1,866 (59.1%) were in DAPSA28, DAPSA66/68 and DAS28-CRP remission at 6 months, respectively. The same eleven predictors were identified for DAPSA28 and DAPSA66/68 remission, while nine predictors were selected for DAS28-CRP remission (Table 2). Eight predictors were common for all three remission outcomes: male gender, longer disease duration and higher CRP were positive predictors, while older age, higher body mass index, patient global score, health assessment questionnaire and 68 tender joint count reduced the odds of remission (Table 2). The same predictors were identified when substituting 66/68-joint counts with 28-joint counts in the set of potential predictors (with 28 tender joint count replacing 68 tender joint count as a predictor).
Conclusion: Identical variables were found as predictors of both DAPSA28 and DAPSA66/68 remission in patients treated with a first TNFi. The majority of these variables also predicted DAS28-CRP remission, although DAS28-CRP remission was more commonly achieved. Our findings suggest that baseline determinants of remission according to different disease activity scores are similar and support the use of DAPSA28 in datasets where only 28-joint counts are available.
To cite this abstract in AMA style:
Georgiadis S, Linde L, Ørnbjerg L, Horskjær Rasmussen S, Askling J, Michelsen B, Di Giuseppe D, Karlsson Wallman J, Glintborg B, Loft A, Bernardes M, Ochôa Matos C, Nordstrom D, Kuusalo L, Moeller B, Nissen M, Gudbjornsson B, Love T, Iannone F, Kvien T, Rotar Z, Castrejon I, Macfarlane G, van de Sande M, Hetland M, Østergaard M. Comparing Predictors of DAPSA28, DAPSA66/68 and DAS28-CRP Remission at 6 Months in Bio-naive Patients with Psoriatic Arthritis Initiating a TNFi in Clinical Practice – Results from the EuroSpA Collaboration [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparing-predictors-of-dapsa28-dapsa66-68-and-das28-crp-remission-at-6-months-in-bio-naive-patients-with-psoriatic-arthritis-initiating-a-tnfi-in-clinical-practice-results-from-the-eurospa/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-predictors-of-dapsa28-dapsa66-68-and-das28-crp-remission-at-6-months-in-bio-naive-patients-with-psoriatic-arthritis-initiating-a-tnfi-in-clinical-practice-results-from-the-eurospa/