Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Disease outcome measures for chronic nonbacterial osteomyelitis (CNO) & Synovitis, Acne, Pustulosis, Hyperostosis and Osteitis (SAPHO) have been proposed and reported but have lacked input and consensus-based confirmation from collaborators other than physicians. OMERACT methodology has been well established to develop core domain sets and corresponding instruments for rheumatic diseases. We aimed to identify candidate domains for a core domain set for patients with CNO & SAPHO following OMERACT filter 2.2.
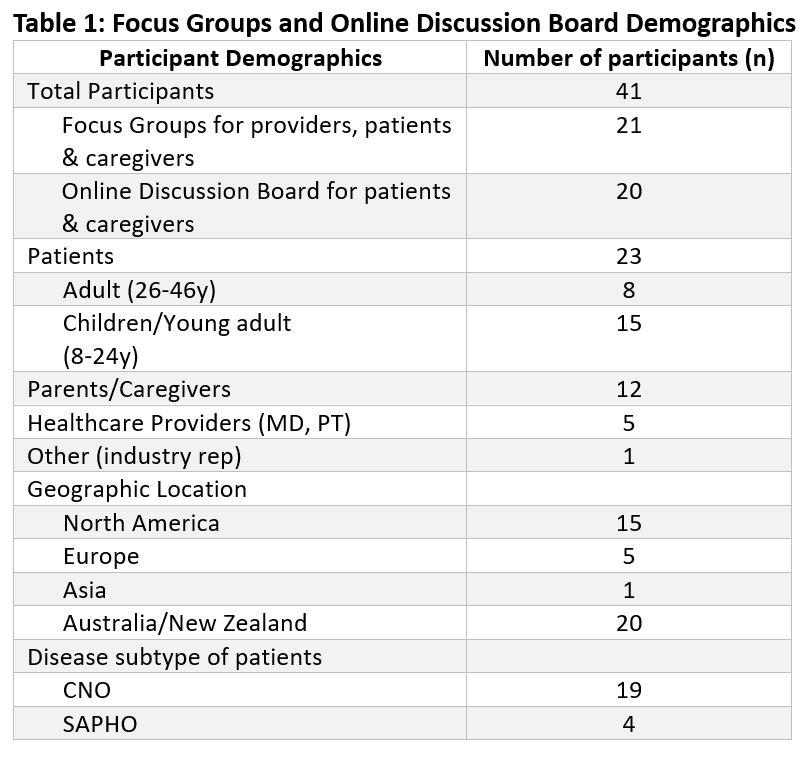
Methods: A scoping review and qualitative research were conducted. The scoping review (SR) was performed by providers using OVID, PubMed, and Google Scholar. Qualitative research consisted of five virtual focus groups from North America, Europe and Asia and four online discussion board groups from Australia and New Zealand. Participants were parents, pediatric patients, adult patients, physicians, a physical therapist and an industry representative. Virtual focus groups (FG) were conducted with a set of questions for participants. Online discussion boards (ODB) used word- or picture-prompted questions to solicit comprehensive responses from patient or caregiver participants. Thematic analysis was used for items identified in the qualitative research. Candidate domains were reviewed and the most representative and informative were chosen through virtual consensus meetings and surveys. Candidate domains were compared between sources and classified into the OMERACT core areas.
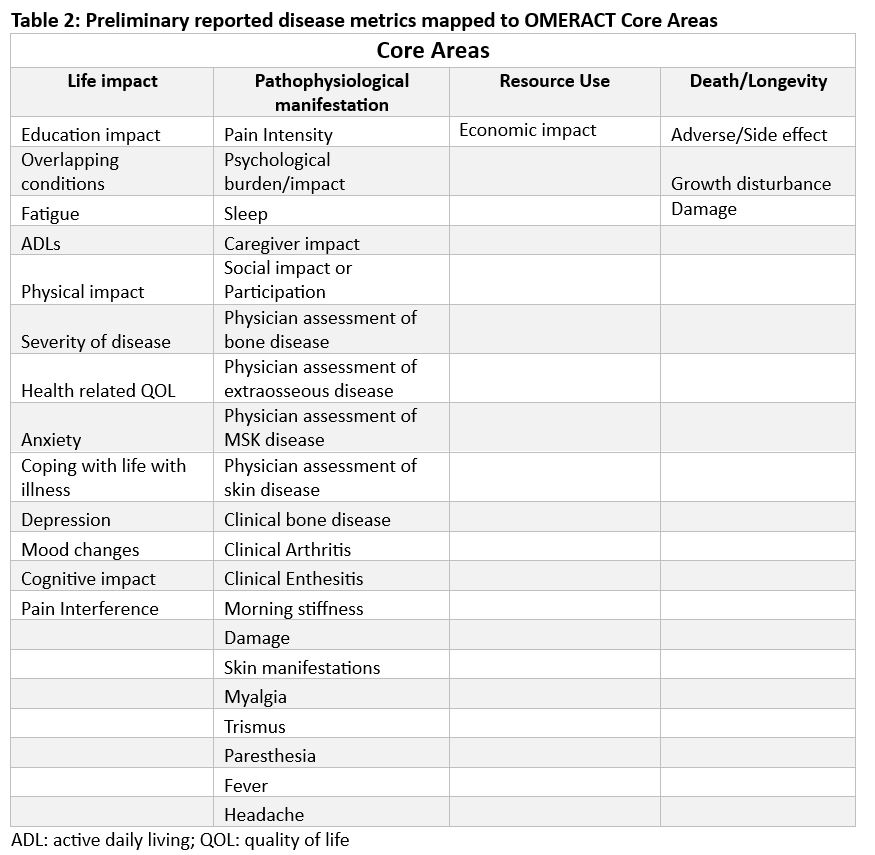
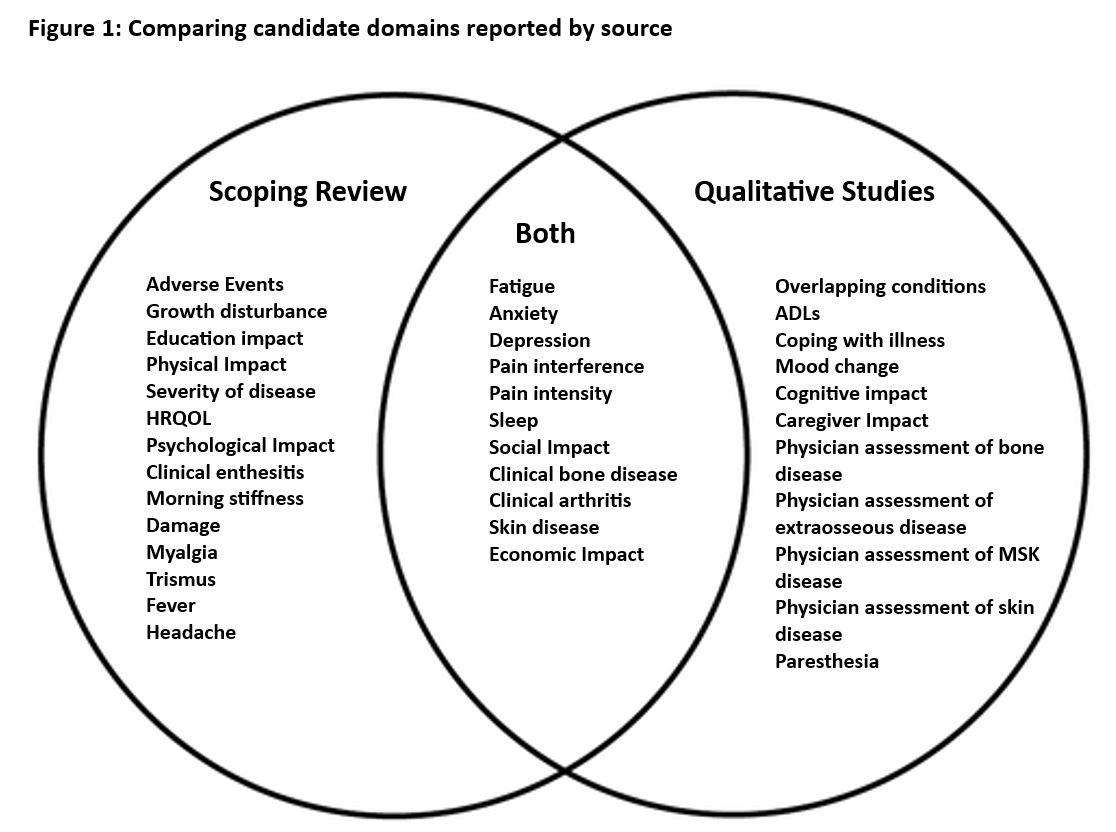
Results: The scoping review identified 260 observational studies published from 1978 -2020. There were 41 participants in the qualitative research (Table 1). Over 300 domains were identified (SR = 220, FG = 79, ODB = 28). These were thematically grouped and reduced to 36 final domains covering each of the OMERACT core areas (Table 2). Most domains map to life impact and pathophysiological manifestations. Twenty-five of the final candidate domains were identified by the scoping review and twenty-two by the qualitative research with 11 items overlapping between the two sources (Figure 1).
Conclusion: A comprehensive and representative preliminary list of domains has been generated for the core domain set for CNO & SAPHO outcome measurement. The next steps include prioritizing these domains through four rounds of Delphi Survey to determine the final lists of core domains, circumstance-dependent core domains, and domains for future consideration. The core domain set will be determined through future work and used to guide future clinical research.
To cite this abstract in AMA style:
Oliver M, Thornhill s, Shea B, Akikusa J, Hedrich C, Mease P, Zhao Y. Comparing Domains Reported by Patients and Other Collaborators in CNO and SAPHO: A Qualitative Study and Scoping Review Using the OMERACT Process [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparing-domains-reported-by-patients-and-other-collaborators-in-cno-and-sapho-a-qualitative-study-and-scoping-review-using-the-omeract-process/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-domains-reported-by-patients-and-other-collaborators-in-cno-and-sapho-a-qualitative-study-and-scoping-review-using-the-omeract-process/