Session Information
Date: Monday, November 14, 2016
Title: Spondylarthropathies Psoriatic Arthritis – Pathogenesis, Etiology - Poster I
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Bone marrow (BM) is not only the place where osteoclast precursors (OCPs) are derived from, but also the major reservoirs of OCPs. Current data suggested that the local inflammatory conditions caused by arthritis progression will induce the proliferation of BM-residing OCPs to further mature into multinucleated mature OC with bone erosion activity, which subsequently expedite arthritis progression. No direct evidence is available to support this notion due to the absence of reliable OCP biomarkers and OCP enumeration with high accuracy. It also remains unclear whether the differentiation of OCPs within BM could be affected solely by skin (psoriasis, Ps) but not bone condition (psoriatic arthritis, PsA). We have previously shown that DC-STAMP, a master regulator in osteoclast differentiation, is elevated in patients with psoriatic arthritis (PsA). In addition, we also showed that DC-STAMP has the potential to serve as a diagnostic biomarker to predict the susceptibility of patients with psoriasis to PsA transition. Given that changes in the BM microenvironment might occur at the early stage of Ps to PsA transition, in this study, we investigate whether Ps pathogenesis could affect the microenvironment of bone marrow for OCP differentiation by comparing DC-STAMP expression level and DC-STAMP+ cell frequency in BM and periphery at healthy and disease status.
Methods:
Bone marrow aspirates (BMA) and blood were collected from 13 Ps patients (PASI score between 35-60) and 5 healthy controls (HC). Total cells or enriched CD14+ monocyte were isolated from BMA and blood, analyzed by 12-color flow cytometry ex vivo, and enumerated OC by TRAP staining after 8-day in vitro culture in OC-promoting media.
Results:
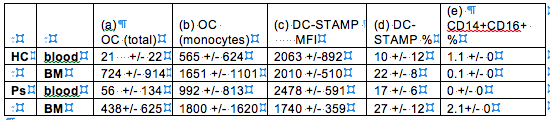
There are 5 major results: (1) More OCPs were found in BM than blood, which is common for both HC and Ps patients (column b); (2) Ps patients have a slightly higher OCPs in BM than HC (1800 +/- 1620 vs. 1651 +/- 1101); (3) Mean Fluorescence Intensity (MFI) of DCSTAMP: This measure the expression level of DC-STAMP. Comparable MFI were found between blood and BM in HC, whereas DC-STAMP MFI is reduced, although not significant, in BM in Ps patients (column C); (4) OCP (purified monocytes) in Ps’s BM is higher than HC (1800 +/- 1620 vs. 1651 +/- 1101, column b). However, the total OCP readouts are lower if monocytes were co-cultured with T & B cells (column a); (5) the inflammatory CD14+CD16+ monocyte subset was elevated in the BM of Ps patients.
Conclusion: (1) Ps pathogenesis induces changes in the BM microenvironment which subsequently affects OCgenesis and OCP frequency within the BM; (2) unidentified cellular factors, such as cell subset & chemokine/cytokines, are present in human bone marrow to suppress OC differentiation (Column A, Ps BM) and DC-STAMP expression (Column C, Ps BM); (3) 3 potential Ps-specific biomarkers are (i) % of CD14+CD16+ cells in BM, (ii) difference between DC-STAMP% in BM and blood (Δ(BM-blood) of DC-STAMP%), and (iii) difference between DC-STAMP MFI in BM and blood (Δ(BM-blood) of DC-STAMP MFI).
To cite this abstract in AMA style:
Chiu YG, Schwarz E, Li D, Huertas N, Bell C, Campbell D, Ritchlin CT. Compare the Potential of Osteoclast Precursors (OCPs) Residing in Bone Marrow and Peripheral Blood As the Surrogates of Psoriasis Pathogenesis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/compare-the-potential-of-osteoclast-precursors-ocps-residing-in-bone-marrow-and-peripheral-blood-as-the-surrogates-of-psoriasis-pathogenesis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/compare-the-potential-of-osteoclast-precursors-ocps-residing-in-bone-marrow-and-peripheral-blood-as-the-surrogates-of-psoriasis-pathogenesis/