Session Information
Date: Monday, November 18, 2024
Title: Abstracts: SpA Including PsA – Diagnosis, Manifestations, & Outcomes II
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Psoriatic arthritis is a chronic systemic inflammatory disorder with significant impacts on health and life quality. Achieving good disease control in psoriatic arthritis (PsA) remains a major challenge even despite recent advancements in targeted therapies. Combining multiple systemic immunomodulatory therapies has been shown to be potentially beneficial and safe in other immune-mediated diseases such as IBD, but data on the current use and safety of targeted combination therapy among patients with PsA in the real world is limited. Mostly case reports and case series exist.
Methods: We utilized longitudinal claims data from a commercial insurance claims database, IBM MarketScan Database, covering 150 million patients in the United States during January 1, 2019-June 15, 2023 to describe combination therapy use in PsA at a population level. For our PsA cohort, we included adults with (i) at least 2 ICD-10 codes of PsA within 12 months that were separated by at least 7 days and less than 365 days and (ii) a diagnosis of PsA followed by 1 or more medication fill instance of a PsA medication. Additionally, we excluded any patients that had other indications for targeted therapy or insurance discontinuation >= 90 days. After finalizing our PsA cohort, we then separated them into a combination therapy cohort (defined as patients that had 3, 4, or 6 consecutive months of medication fill data for 2 or more different non-csDMARD drug classes) and a non-combination therapy cohort. We compared frequency of ICD-10 codes for serious or opportunistic infections requiring inpatient level of care in both cohorts.
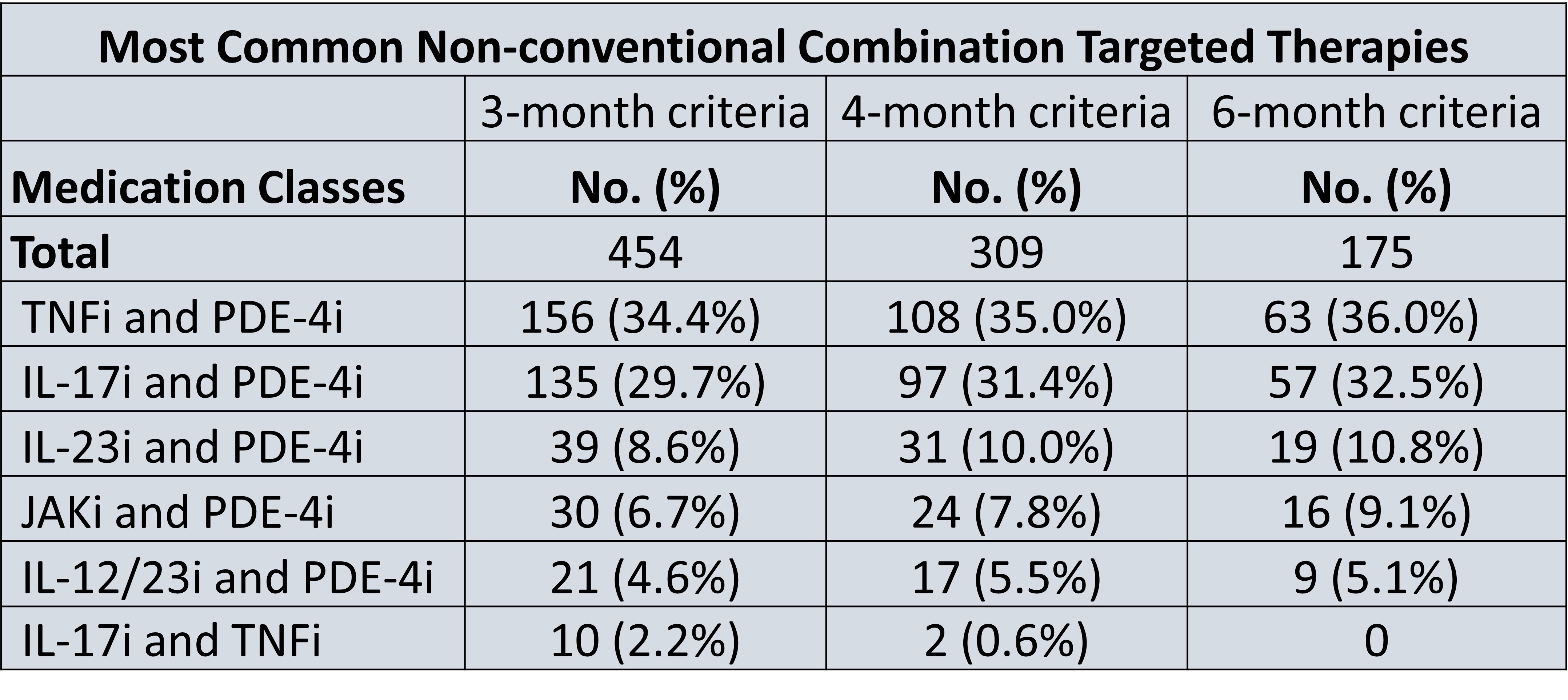
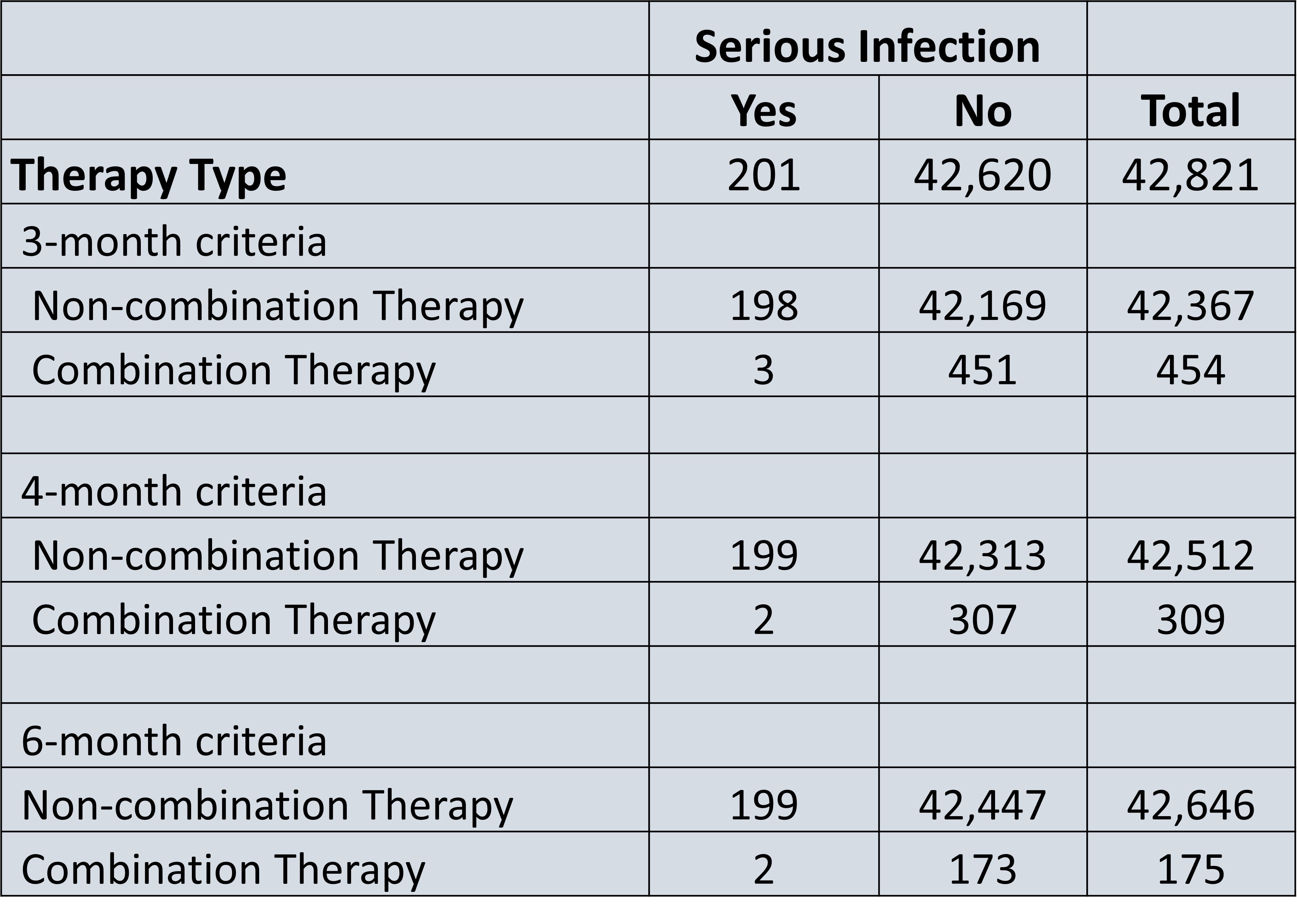
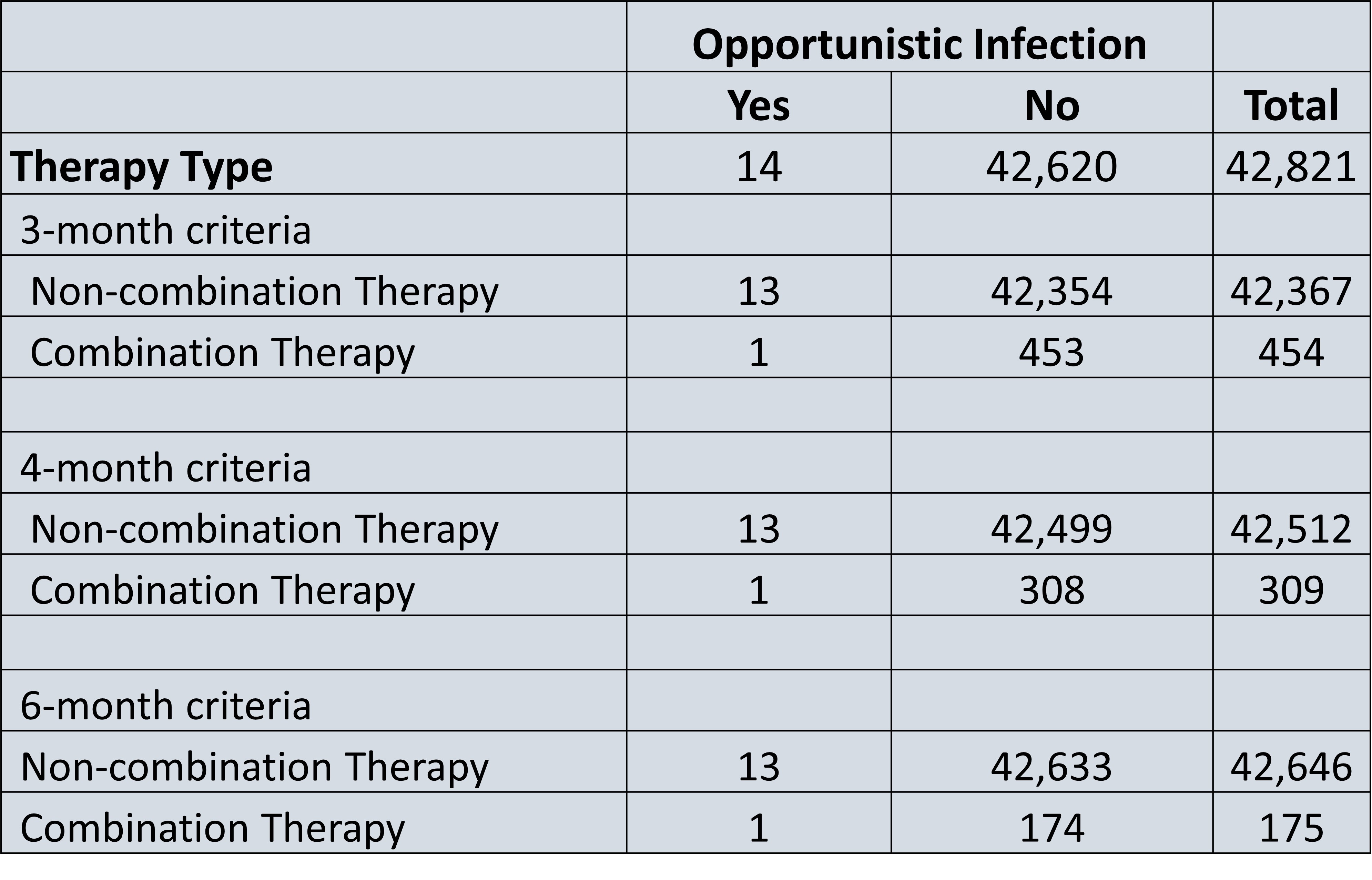
Results: A total of 42,821 patients had PsA, with a total of 454 patients or 1.1% of all patients with PsA on non-conventional combination targeted therapy for a consecutive 3 months. 309 (0.7%) patients were on combination targeted therapy for a consecutive 4 months, and 175 (0.4%) patients were on combination targeted therapy for a consecutive 6 months. The two most common combination therapy drug classes in all 3 cohorts was a PDE-4 inhibitor and a TNF-alpha inhibitor (34-36%), followed by an IL-17 inhibitor and PDE-4 inhibitor (30- 33%) (Figure 1). The risk of serious infection among non-combination therapy patients was 4.66 risk per 1000 patients, while the risk of serious infection among combination therapy patients ranged from 6.61-9.95 risk per 1000 patients, representing a 1.41-2.45-fold increase in risk (Figure 2). Furthermore, the risk of opportunistic infection among non-combination therapy patients ranged was 0.30 risk per 1000 patients, while the risk of serious infection among combination therapy patients ranged from 2.2-11.43 risk per 1000 patients (Figure 3).
Conclusion: In commercially insured adults with PsA on non-conventional combination targeted therapy, we found a higher unadjusted incidence of serious bacterial and opportunistic infections that required hospitalization compared to standard therapy. Numerically, the rate of infections was still low and similar to that of other studies. Limitations of this study include small sample size, challenging bias/confounding by indication, retrospective design, and inability to adjust for PsA severity.
To cite this abstract in AMA style:
Wu A, Zhang A, Guo Y, Liu J, Yang D, Perez Chada L, Ogdie A, Scher J, Merola J. Comparative Safety and Use of Non-conventional Combination Targeted Therapy in Adults with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparative-safety-and-use-of-non-conventional-combination-targeted-therapy-in-adults-with-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-safety-and-use-of-non-conventional-combination-targeted-therapy-in-adults-with-psoriatic-arthritis/