Session Information
Date: Friday, November 6, 2020
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a heterogeneous condition; this presents a challenge in how to best measure disease activity for all patients. While several outcome measures have been studied for use in traditional randomized clinical trials (RCT) of PsA, this has been done in the context of pre-defined inclusion criteria derived from a relatively homogenous population with a polyarticular phenotype and higher disease activity than is typically encountered in “real world” practice. There remains a need to determine the ideal outcomes for PsA pragmatic trials that enroll patients representative of those seen in routine care in the clinics. We aimed to investigate the responsiveness of outcome measures among patients with PsA initiating therapy in clinical practice.
Methods: Patients were enrolled in the Psoriatic Arthritis Research Consortium (PARC), a multi-center longitudinal observational cohort study, between 2017-2020. We collected data at initiation of a new treatment for PsA, including a switch in treatment, and follow-up at approximately 16 weeks. At both visits, patients completed patient-reported outcome assessments (PROs) and rheumatologists completed a comprehensive musculoskeletal exam. Change in scores were calculated for the following measures between both timepoints: Routine Assessment of Patient Index Data (RAPID3), clinical Disease Activity of Psoriatic Arthritis (cDAPSA), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), PsA Impact of Disease (PSAID), Multi-Dimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire (HAQDI), Patient Reported Outcome Measure Information System (PROMIS) physical health (PH) subscore, PROMIS mental health (MH) subscore, PROMIS fatigue, PROMIS depression, patient pain assessment, patient global assessment, physician global assessments of joints and overall, and tender (68) and swollen (66) joint counts. Standardized response means (SRMs) were calculated as the mean change between two visits divided by the standard deviation. Responsiveness cutoffs are debatable, but SRMs may roughly be considered large at >0.8, moderate 0.5-0.8, and small < 0.5.
Results: Among 177 unique patients, 266 therapy courses met inclusion criteria. The mean age was 51 years, 53% were female, mean BMI was 29.9, and 16% had axial disease. Among the 266 visits, 145 initiated a TNFi, 55 an IL17i, 96 an OSM, and 14 started another biologic or JAKi. Mean swollen and tender joint counts (66/68) at therapy initiation were 2.9 and 5.8. Responsiveness for all measures was small to moderate (Figure 1.) BASDAI was the most responsive, and tender joint count the least responsive; SRMs were similar among those initiating a biologic therapy vs. any therapy.
Conclusion: Patients with PsA switching or starting therapy in this patient population had different clinical characteristics compared to those usually enrolling in a RCT. Similarly, responsiveness of outcome measures in clinical practice looks different than traditional PsA clinical trials, with SRMs that are generally lower. This data will be useful for selecting outcome measures and calculating sample size for pragmatic trials in PsA.
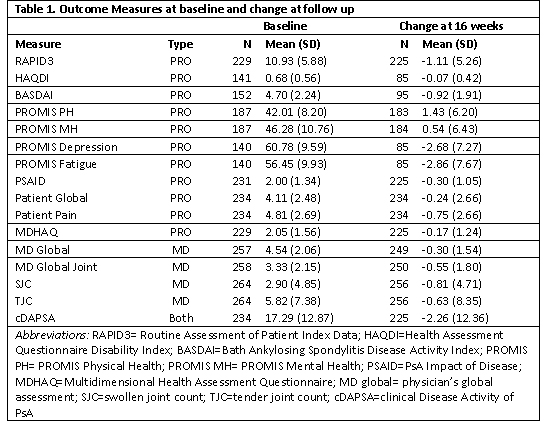 Table 1. Baseline and change scores
Table 1. Baseline and change scores
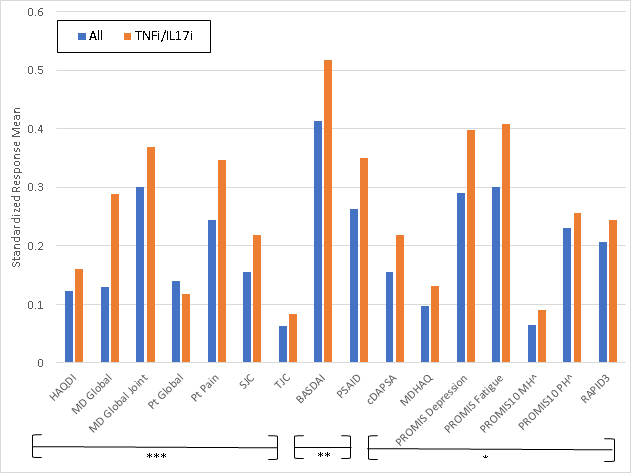 Figure 1. Standardized Response Means of Instruments Tested. ***=Outcome measures frequently used in randomized controlled trials (RCTs) for PsA **= Outcome measures sometimes used in RCTs for PsA *= Outcome measures rarely used in RCTs for PsA Abbreviations: HAQDI=Health Assessment Questionnaire Disability Index; MD global= physician’s global assessment; SJC=swollen joint count; TJC=tender joint count; BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; PSAID=PsA Impact of Disease; ; cDAPSA=clinical Disease Activity of PsA; MDHAQ=Multidimensional Health Assessment Questionnaire; PROMIS MH= PROMIS Mental Health; PROMIS PH= PROMIS Physical Health; RAPID3= Routine Assessment of Patient Index Data
Figure 1. Standardized Response Means of Instruments Tested. ***=Outcome measures frequently used in randomized controlled trials (RCTs) for PsA **= Outcome measures sometimes used in RCTs for PsA *= Outcome measures rarely used in RCTs for PsA Abbreviations: HAQDI=Health Assessment Questionnaire Disability Index; MD global= physician’s global assessment; SJC=swollen joint count; TJC=tender joint count; BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; PSAID=PsA Impact of Disease; ; cDAPSA=clinical Disease Activity of PsA; MDHAQ=Multidimensional Health Assessment Questionnaire; PROMIS MH= PROMIS Mental Health; PROMIS PH= PROMIS Physical Health; RAPID3= Routine Assessment of Patient Index Data
To cite this abstract in AMA style:
Stull C, Reddy S, Husni M, Scher J, Craig E, Walsh J, Ogdie A. Comparative Responsiveness of Outcome Measures in Psoriatic Arthritis: Preparation for Pragmatic Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparative-responsiveness-of-outcome-measures-in-psoriatic-arthritis-preparation-for-pragmatic-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-responsiveness-of-outcome-measures-in-psoriatic-arthritis-preparation-for-pragmatic-trials/
