Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Belimumab and anifrolumab, biologic therapies, have shown to improve systemic lupus erythematosus (SLE) outcomes in clinical trials, but there is a lack of real-world comparative safety data. This study aims to fill this gap by leveraging the TriNetX Research Network, which aggregates electronic medical records from diverse healthcare organizations, allowing for robust statistical analyses and enhanced generalizability. The study aims to compare adverse events, including mortality, sepsis, herpes zoster, dialysis, major adverse cardiovascular events (MACE), pulmonary embolism (PE), venous thromboembolism (VTE), COVID-19, upper respiratory infections (URI), pneumonia, and mental illness, in SLE patients treated with belimumab versus anifrolumab.
Methods: The study identified 489 and 9022 SLE patients who initiated anifrolumab or belimumab. After excluding those treated with the other biologic within 3prior 30 days or initiated biologic before August 2021, and matching the two groups by sex, age and index year at a ratio of 1:2, we included 392 anifrolumab users and 784 belimumab users. Diagnoses of SLE, comorbidities, and outcomes were primarily based on ICD-10-CM or procedure codes.
To investigate outcomes related to chronic conditions such as dialysis and mental illness, patients with these conditions within one year before the index date were excluded. The censored dates were 90 days after the last dose of biologics, the date of switching to the other biologic, the date of death, or the last date of the dataset (i.e., December 5, 2023), whichever occurred first.
After adjusting for potential confounders, including age, sex, race, history of herpes zoster vaccination, comorbidities including diabetes, hypertension, malignancies, diseases of arteries, arterioles and capillaries, and spondyloarthritis, and use of medications including rituximab, leflunomide, methotrexate, hydroxychloroquine, mycophenolic acid/mycophenolate mofetil, calcineurin inhibitors, azathioprine, corticosteroid and non-steroidal anti-inflammatory drugs within one year before the index date, the relative risks of various adverse events in anifrolumab-treated SLE patients compared with belimumab-treated SLE patients were estimated using multivariable Cox regression analyses, presented as adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs).
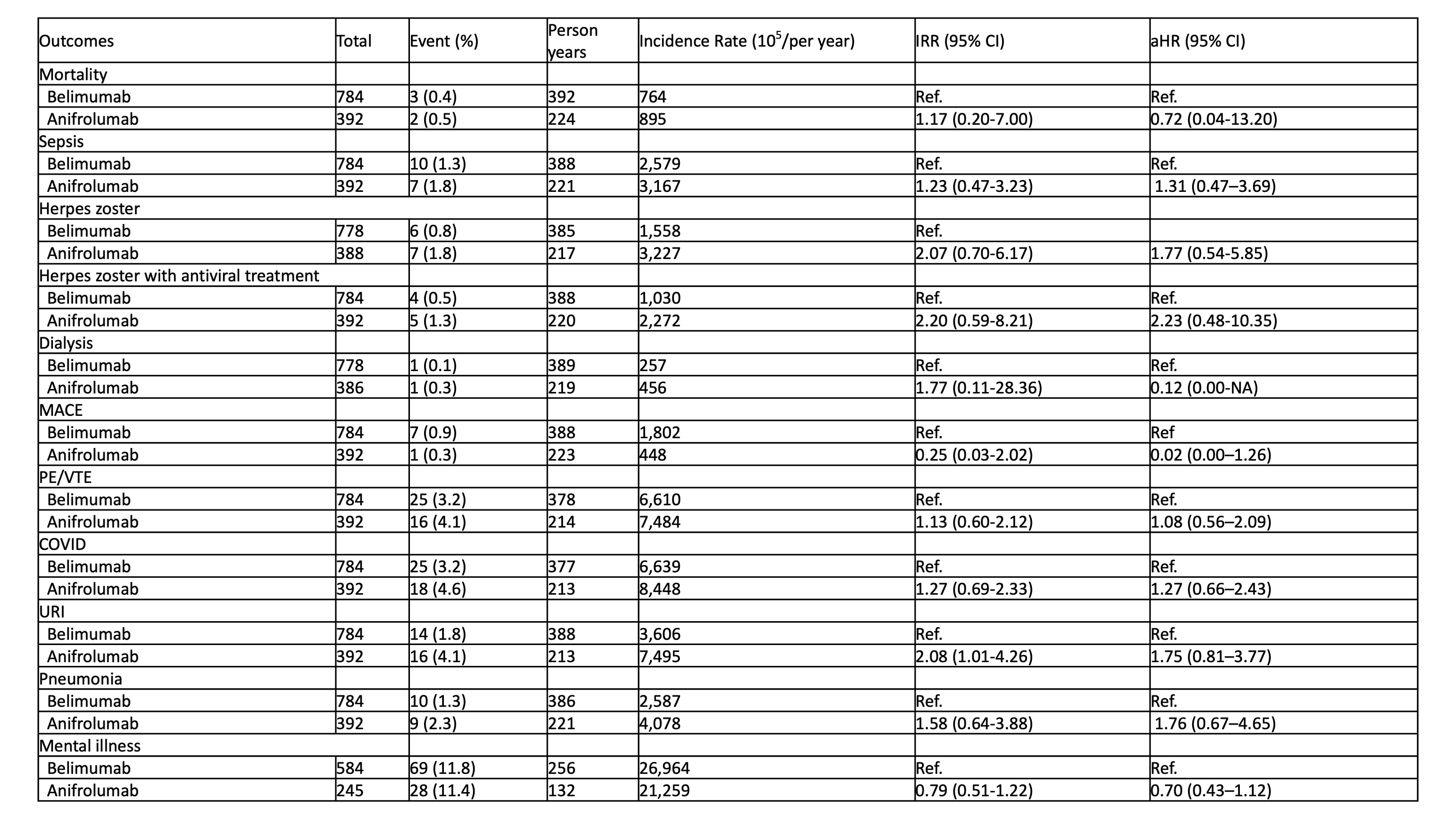
Results: The mean ± standard deviation (SD) age was 44.6±12.3 years, and the proportion of women was 97.7% in both groups. As shown in Table 1, the incidence of URI was significantly higher in anifrolumab users than in belimumab users. However, after adjusting for potential confounders, the risks of mortality, sepsis, herpes zoster, dialysis, MACE, PE/VTE, COVID-19, URI, pneumonia, and mental illness were comparable between both groups.
Conclusion: This real-world global multicenter matched cohort study demonstrated that the risks of adverse events were not significantly different between SLE patients treated with anifrolumab or belimumab.
Disclosures: H. Chen: None.
To cite this abstract in AMA style:
Chen H. Comparative Harms in Patients with Systemic Lupus Erythematosus Treated with Anifrolumab or Belimumab: A Multicenter Cohort Study Using the TriNetX Research Network [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparative-harms-in-patients-with-systemic-lupus-erythematosus-treated-with-anifrolumab-or-belimumab-a-multicenter-cohort-study-using-the-trinetx-research-network/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-harms-in-patients-with-systemic-lupus-erythematosus-treated-with-anifrolumab-or-belimumab-a-multicenter-cohort-study-using-the-trinetx-research-network/