Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: The first subcutaneous (SC) formulation of infliximab (IFX) received approval for the treatment of rheumatoid arthritis (RA), Crohn’s disease (CD), ulcerative colitis (UC), ankylosing spondylitis (AS), psoriatic arthritis (PsA) and psoriasis (PsO) in adults. Pivotal studies of SC IFX (Westhovens R, 2021; Schreiber S, 2021) demonstrated the efficacy and safety of switching from IV to SC IFX for patients with RA, UC and CD.
To improve confidence and uptake for SC IFX, the real-world safety profile continues to be needed. The purpose of the study is to describe and assess adverse events (AEs) associated with SC IFX and IV IFX in all indications using a pharmacovigilance database.
Methods: Data on case reports with SC IFX and IV IFX were obtained from the Celltrion’s global safety database (22 Nov 2019 ~ 7 Feb 2022) since the first launch of SC IFX. AEs reported for SC IFX and IV IFX at the primary system organ class (SOC) level were compared. Disproportionality analysis was conducted. The reporting odds ratio (ROR) and its 95% CI were calculated.
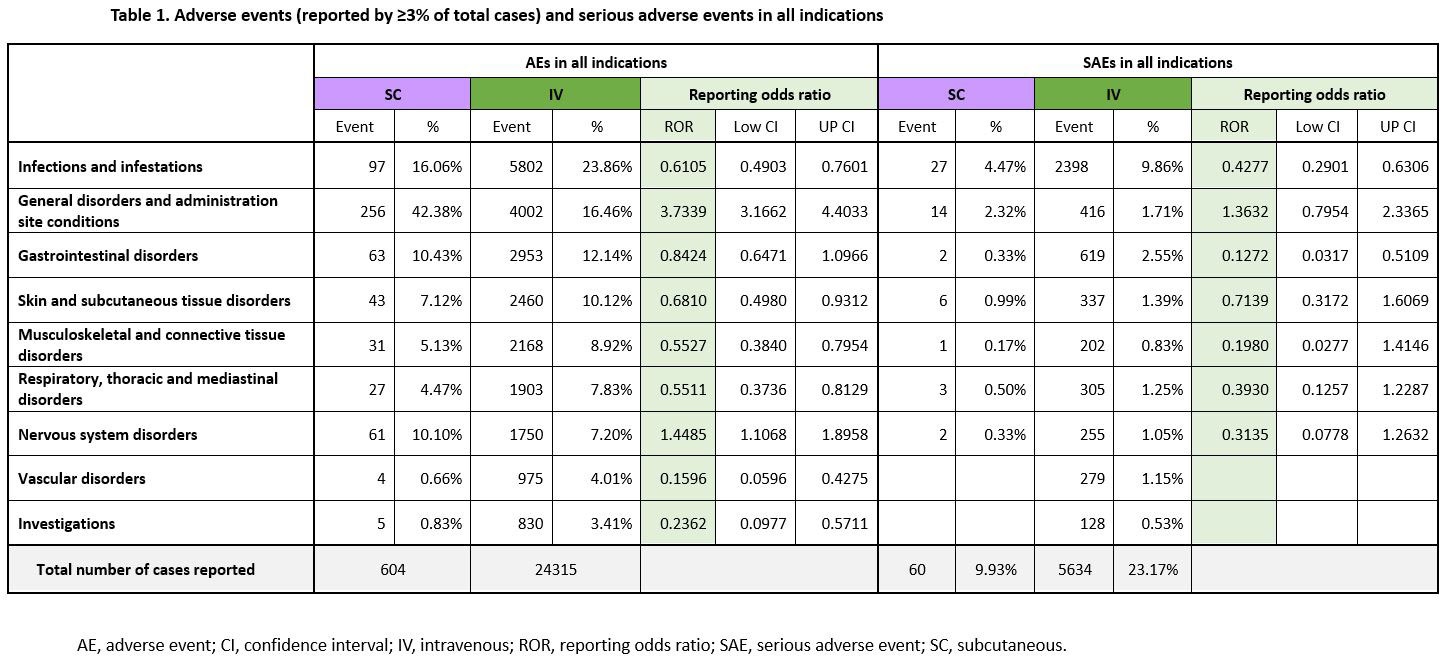
Results: A total of 24,919 AEs were identified. Of these, 604 (2.4%) events for SC IFX and 24,315 (97.6%) events for IV IFX were reported (Table 1).
SC IFX was associated with a low reporting of AEs belonging to the SOCs “Infections and infestations”, “Skin and subcutaneous tissue disorders”, “Musculoskeletal and connective tissue disorders”, “Respiratory, thoracic and mediastinal disorders”, “Vascular disorders” and “Investigations”.
There was a higher reporting probability of SC IFX versus IV IFX for AEs with the SOCs “General disorders and administration site conditions” and “Nervous system disorders”. In each SOC, “injection site reaction (ISR)” and “headache” were most commonly reported. However, most of the AEs were not serious, and no significant difference was found between the two groups in the reporting frequency of serious adverse events (SAE) in both SOCs.
SC IFX showed a lower reporting probability of SAEs in “infection and infestations” and “Gastrointestinal disorders” versus IV IFX. For the remainder of SOCs, no substantial differences were observed.
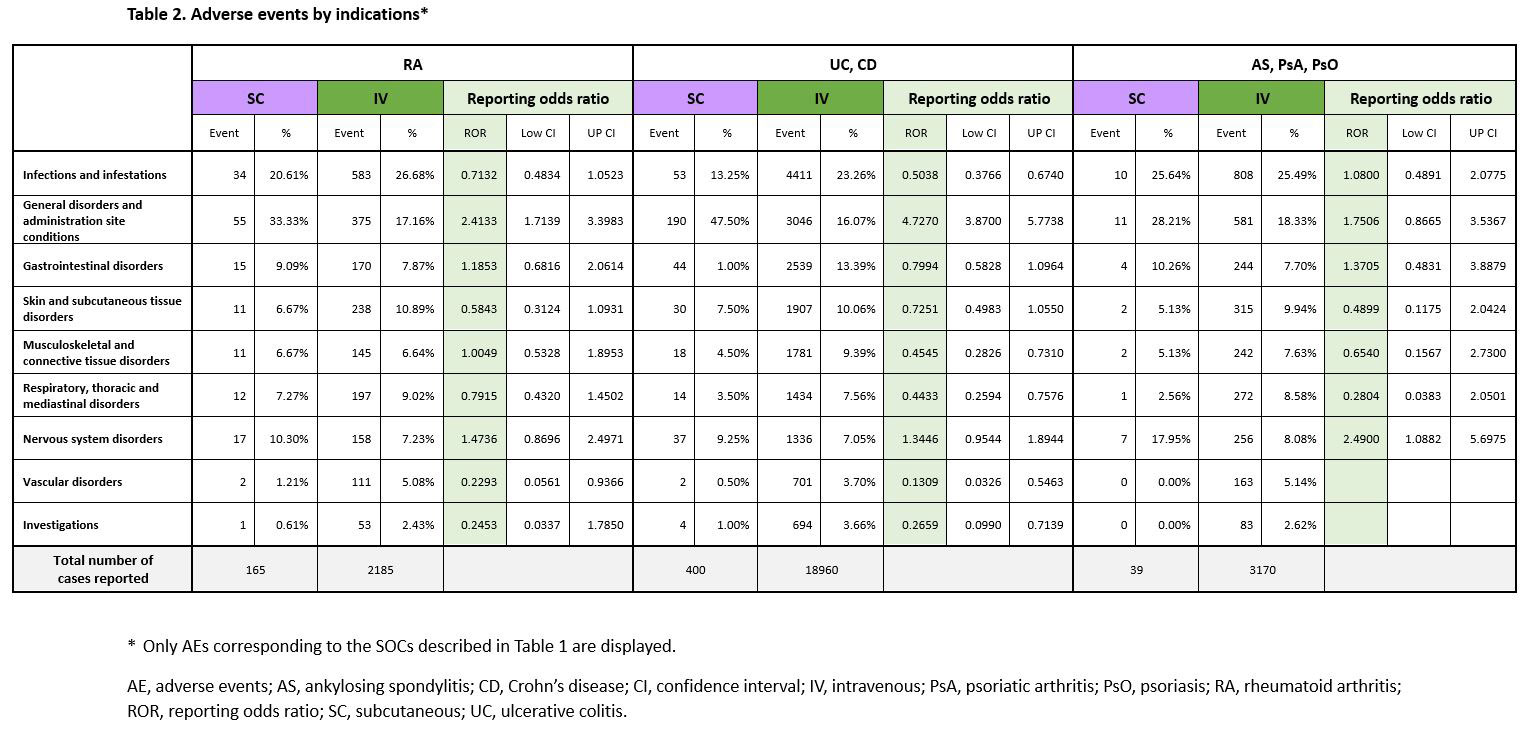
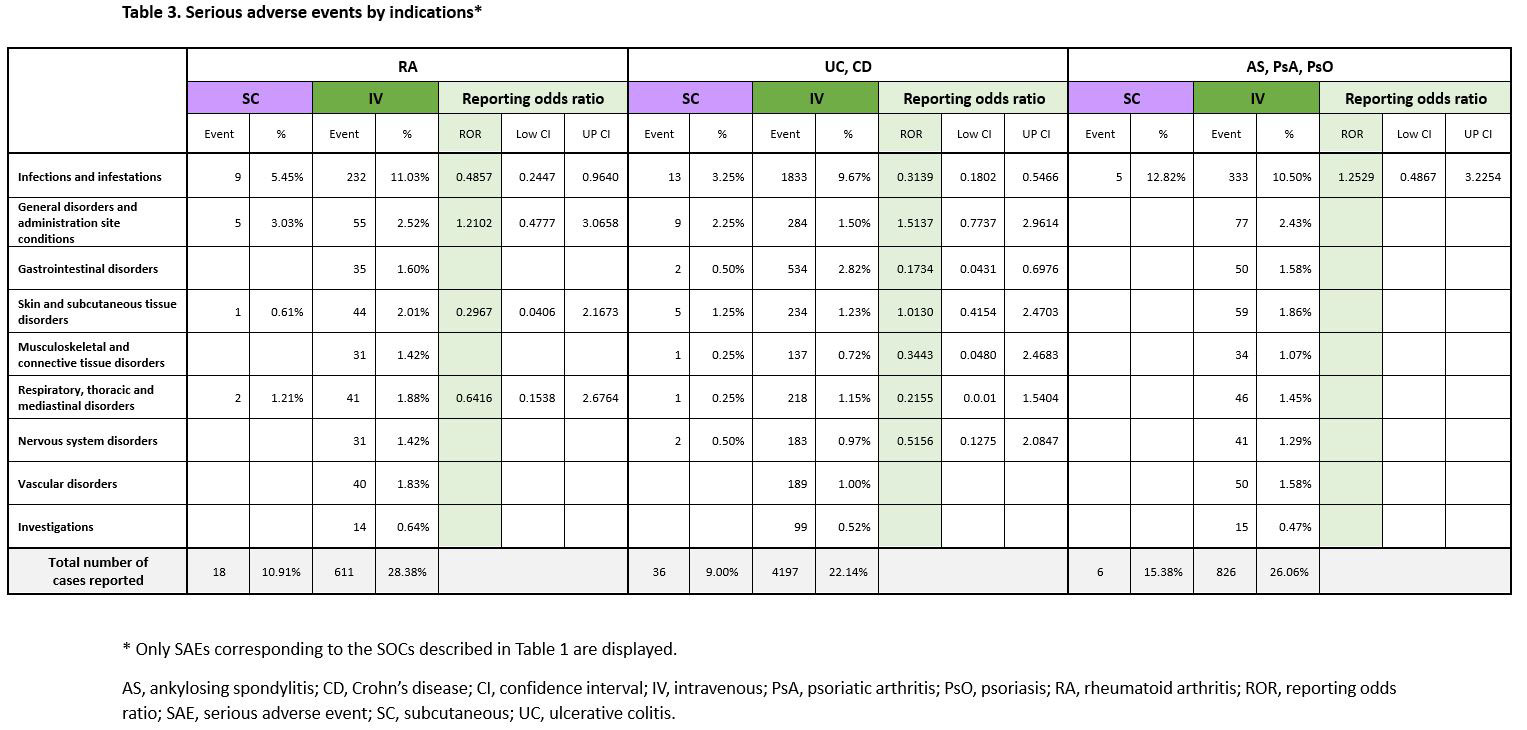
Disproportionality analyses by indications also showed similar trends (Table 2). Overall, the proportion of SAEs reported by each indication was lower in the SC group (Table 3). The ROR in most SOCs was not significantly different.
Conclusion: In most of the SOCs, the reporting probabilities of AEs and SAEs for SC IFX SC versus IV IFX were found to be lower or similar. This was similar in the analysis by indication.
An increased reporting of AEs for SOC “General disorders and administration site conditions” was observed with SC IFX versus IV IFX, due to the frequent reporting of ISRs in SC IFX. This is expected due to the difference in the route of administration, which is consistent with the results of pivotal clinical trials. Serious ISRs were low in both groups.
Although the characteristics of patients were not balanced and the number of reports collected in the SC group was relatively small, this real-world analysis confirms a comparable safety profile of SC IFX and IV IFX in the licensed indications.
To cite this abstract in AMA style:
Baraliakos X, Kyburz D, Avouac J, Barkham N, Lee S. Comparative Evaluation of Adverse Events Associated with Subcutaneous Infliximab (CT-P13 SC) and Intravenous Infliximab: A Real-world Analysis of Post-marketing Surveillance Data [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/comparative-evaluation-of-adverse-events-associated-with-subcutaneous-infliximab-ct-p13-sc-and-intravenous-infliximab-a-real-world-analysis-of-post-marketing-surveillance-data/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-evaluation-of-adverse-events-associated-with-subcutaneous-infliximab-ct-p13-sc-and-intravenous-infliximab-a-real-world-analysis-of-post-marketing-surveillance-data/