Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To evaluate the efficacy, safety, and tolerability of the antifibrotic agents nintedanib and pirfenidone in the treatment of interstitial lung disease (ILD) associated with rheumatoid arthritis (RA), systemic sclerosis (SSc), and other systemic autoimmune diseases (SADs) fulfilling current criteria for progressive pulmonary fibrosis (PPF).
Methods: A multicentre, longitudinal retrospective observational study was conducted. Data collected included pulmonary function test (PFT) results, adverse events (AEs), tolerability, and drug retention
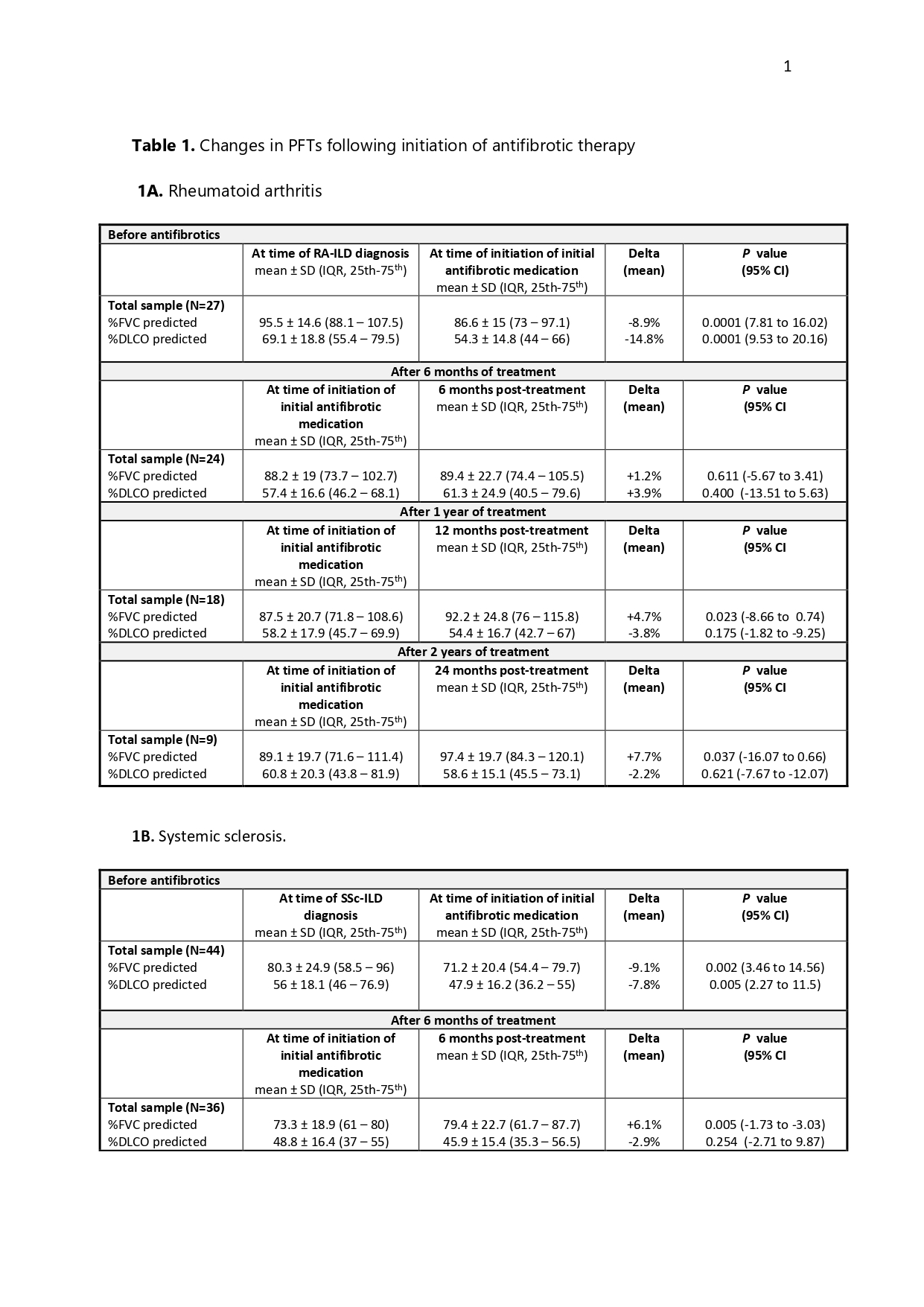
Results: A total of 132 patients were included (74 women): 27 with RA-ILD (20.5%), 44 with SSc-ILD (33%), and 61 with SADs-ILD (46.5%), including 24 with IPAF, 10 with antisynthetase syndrome, 9 with primary Sjögren’s syndrome, 7 with ANCA-associated isolated ILD, 6 with ANCA-associated vasculitis, 3 with SLE, and 2 with sarcoidosis.Of these, 116 (87.9%) started nintedanib and 16 (12.1%) pirfenidone. The UIP pattern was present in 47.7% (63). Eighty-five percent (112) received combination therapy with a bDMARD, a csDMARD/immunosuppressant, or both. The median post-antifibrotic follow-up was 25 months (IQR 25th–75th: 14–46).Changes in PFTs following initiation of antifibrotic therapy are summarised in Table 2. Across the entire study population, significant declines in PFTs were observed prior to antifibrotic initiation. In patients with RA-ILD, antifibrotic treatment was associated with a significant increase in %CVF at 12 months (+4.7%, p=0.023) and 24 months (+7.7%, p=0.037), while %DLCO remained stable (−3.8% and −2.2%; p=0.175 and p=0.621, respectively). In the SSc-ILD group, %CVF improved consistently (+8.7% at both 12 and 24 months; p=0.024 and p=0.018), whereas %DLCO showed a mild, non-significant decrease (−2.1% and −1.8%; p=0.560 and p=0.427).In contrast, among patients with other SADs-ILD, %CVF remained stable at 12 months (−0.7%, p=0.665) and declined slightly at 24 months (−3.2%, p=0.103), whereas %DLCO decreased progressively (−3.7% and −5.1%; p=0.422 and p=0.025).Data on safety and tolerability are summarised in Table 2. AEs were common across all groups: 81.5% in RA-ILD, 75% in SSc-ILD, and 65.6% in SADs-ILD. Permanent dose reductions due to AEs were more frequent with nintedanib than pirfenidone.Permanent treatment discontinuation was observed in 52.3% of SSc-ILD, 37% of RA-ILD, and 36.1% of SADs-ILD patients. Discontinuation due to AEs occurred in 31.8%, 18.5%, and 14.8%, respectively. Although these figures suggest lower tolerability in the SSc-ILD subgroup, differences were not statistically significant in the overall comparison (p=0.100) or in pairwise analyses: p=0.276 for SSc-ILD vs RA-ILD, p=0.055 for SSc-ILD vs SADs-ILD, and p=0.754 for RA-ILD vs other SADs-ILD
Conclusion: Across the three groups, antifibrotic treatment resulted in either PFT stabilisation or a reduced rate of decline. Patients with SSc-ILD appeared to have lower tolerability, but no statistically significant differences were observed.
To cite this abstract in AMA style:
Narváez J, Barrios O, Maymó-Paituvi P, ALEGRE SANCHO J, Castellví I, Vicens Zygmunt V, Bermudo G, De Daniel Bisbe L, Aguilar-Coll M, Roig Kim M, Nolla J, Molina-Molina M. Comparative efficacy, safety and tolerability of antifibrotic therapies across systemic autoimmune diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparative-efficacy-safety-and-tolerability-of-antifibrotic-therapies-across-systemic-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-efficacy-safety-and-tolerability-of-antifibrotic-therapies-across-systemic-autoimmune-diseases/

.jpg)
.jpg)