Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Systemic Lupus Erythematosus – Treatment II (2693–2698)
Session Type: Abstract Session
Session Time: 12:45PM-1:00PM
Background/Purpose: The efficacy of calcineurin inhibitors in the induction treatment of lupus nephritis (LN) has been increasingly supported by recent studies. Based on these findings, current guidelines recommend a combination of tacrolimus (TAC), mycophenolate mofetil (MMF), and glucocorticoids (GC) as an option for the first-line induction therapy. However, several studies have also demonstrated that TAC + GC therapy is effective for induction therapy, with favorable safety profile. Therefore, our study aimed to compare the efficacy of TAC + GC versus TAC + MMF + GC for remission induction in patients with proliferative LN.
Methods: This multicenter retrospective cohort study, designed as a target trial emulation, included patients with biopsy proven proliferative LN who received either TAC + GC or TAC + MMF + GC as induction therapy between September 2007 and July 2023. Based on the induction regimen, patients were classified into the TAC + GC and TAC + MMF + GC groups. The primary outcome was 12-month total renal response, defined as achieving either complete or partial renal response after 12 months. To address the baseline imbalances between the two groups, inverse probability of treatment weighting (IPTW) was applied. The comparative efficacy of TAC + MMF + GC compared to TAC + GC was estimated by using binary logistic regression, based on both as-started (corresponding to intention-to-treat) and on-treatment (corresponding to per-protocol) analyses.
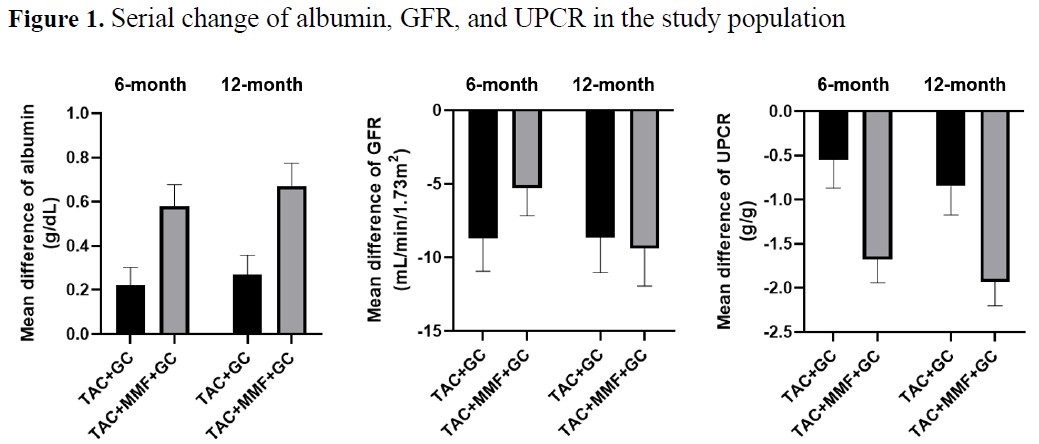
Results: A total of 115 patients (48 in TAC + GC group and 67 in TAC + MMF + GC group) were included in the analysis. The baseline characteristics of the study population is shown in Table 1. The 12-month total renal response was achieved in 16 (33.3%) patients in the TAC + GC group and 40 (59.7%) patients in the TAC + MMF + GC group. Both groups showed improvements in serum albumin and proteinuria, while renal function declined (Figure 1). Compared to the TAC + GC group, the TAC + MMF + GC group showed significantly higher 12-month total renal response in both as-started and on-treatment analyses (Table 2). Adverse drug reactions occurred in 7 (14.6%) patients in the TAC + GC group and 11 (16.4%) patients in the TAC + MMF + GC group.
Conclusion: Compared to TAC + GC, TAC + MMF + GC therapy showed significantly higher renal response and comparable adverse drug reactions in patients with proliferative LN. These findings support TAC + MMF + GC as the preferred TAC-based induction regimen for proliferative LN.
To cite this abstract in AMA style:
Kim Y, Lee H, Park J, Lee E, Ha Y, Park J. Comparative Efficacy of Tacrolimus-Based Induction Therapy with and without Mycophenolate Mofetil in Lupus Nephritis: A Target Trial Emulation Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparative-efficacy-of-tacrolimus-based-induction-therapy-with-and-without-mycophenolate-mofetil-in-lupus-nephritis-a-target-trial-emulation-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-efficacy-of-tacrolimus-based-induction-therapy-with-and-without-mycophenolate-mofetil-in-lupus-nephritis-a-target-trial-emulation-study/

.jpg)
.jpg)