Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: To compare ACR responses of novel DMARDs as monotherapy or in combination with methotrexate (MTX), including subcutaneous (SC) abatacept and SC tocilizumab (TCZ), in RA patients with an inadequate response to conventional DMARDs (DMARD-IR).
Methods: A systematic literature review identified randomized clinical trials (RCTs) that evaluated abatacept (intravenous [IV] and SC), anakinra, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, tofacitinib, and TCZ (IV and SC). Thirty RCTs were identified. Reported treatment effects in terms of ACR responses at 24 weeks were synthesized by means of Bayesian network meta-analyses to allow comparisons of the different treatments as monotherapy and combination therapy. Based on previous reviews1,2 an assumption was made that the effects of anti-tumor necrosis factor (aTNF) therapy were exchangeable. Given this, and the limited data identified for these therapies in monotherapy, aTNF data were pooled.
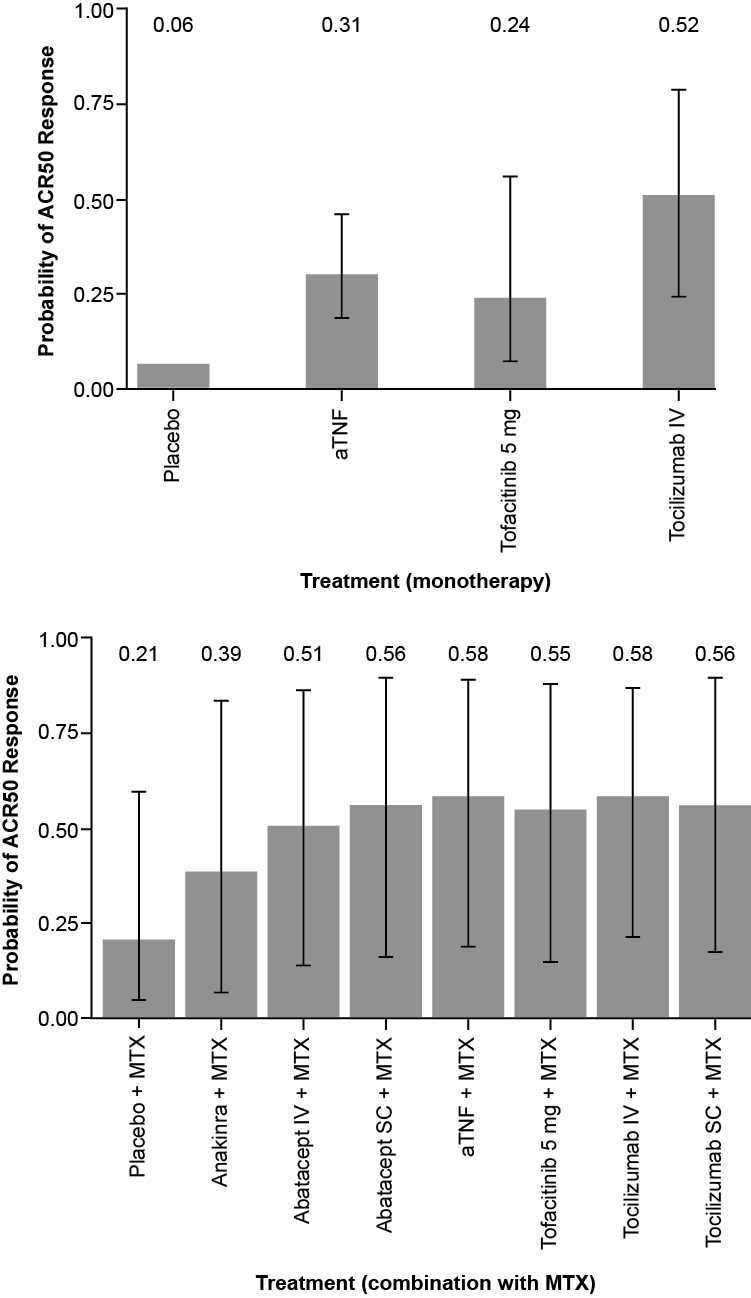
Results: aTNFs + MTX, tofacitinib + MTX, abatacept IV/SC + MTX, and TCZ IV/SC + MTX demonstrated comparable ACR responses while anakinra + MTX was less efficacious. Among biologic monotherapies, greater ACR20/50/70 responses were observed with TCZ IV than with aTNFs and tofacitinib (Figure 1). When comparing biologics + MTX with biologic monotherapies, ACR20, ACR50, and ACR70 responses with TCZ + MTX were similar to TCZ as monotherapy (OR=1.04, 95% CI, 0.39-2.80; OR=1.28, 95% CI, 0.46-3.51; OR=0.97, 95% CI, 0.38-2.49, respectively), whereas with aTNF + MTX greater ACR20/50/70 responses were observed than with aTNF monotherapy (OR=2.22; 95% CI, 0.46-10.83, probability better=84%; OR=3.12, 95% CI, 0.60-16.32, probability better=92%; OR=1.39, 95% CI, 0.26-6.78, probability better=68%, respectively). For tofacitinib, sensitivity analyses showed conflicting results for the indirect comparison of tofacitinib + MTX versus tofacitinib.
Conclusion: Results of this meta-analysis suggest that most available novel DMARDs, in combination with MTX, have similar levels of efficacy in DMARD-IR patients. As monotherapy, TCZ is likely to have a greater response than aTNFs and tofacitinib. TCZ monotherapy also shows comparable efficacy compared to TCZ + MTX, whereas aTNFs in combination with MTX showed greater ACR responses compared with aTNF monotherapy at 24 weeks.
References: 1. Nixon R Rheumatology. 2007;46:1140; 2. Salliot Ann Rheum Dis. 2011;70:266.
Figure. Expected ACR50 response at 24 weeks with monotherapy (top) or combination therapy (bottom) (random effects network meta-analysis)
Disclosure:
F. Buckley,
Roche Pharmaceuticals,
5;
A. Finckh,
BMS, Pfizer;,
2,
BMS, Abbott, Roche, Pfizer,
5,
Roche Pharmaceuticals,
8;
T. W. J. Huizinga,
Abbott, Axis Shield Diagnostics, Biotest AG, BMS, Crescendo Bioscience, Roche, Novartis, Schering-Plough, UCB, Wyeth, Pfizer,
5;
F. Dejonckheere,
F. Hoffmann-La Roche,
3;
J. P. Jansen,
Roche Pharmaceuticals,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-efficacy-of-novel-disease-modifying-antirheumatic-drugs-as-monotherapy-and-in-combination-with-methotrexate-in-rheumatoid-arthritis-patients-with-an-inadequate-response-to-traditional-dise/