Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Active ankylosing spondylitis (AS) has been associated with poor quality of life and work disability in up to 50% of patients (1). There is an unmet medical need for treatment of patients with active axial disease who have an inadequate response to biologic DMARDs. Janus Kinase (JAK) inhibitors are not currently approved for use in active AS, but several clinical trials suggest their efficacy. Network meta-analyses (NMA) have been conducted to evaluate relative efficacy of JAK inhibitors and biologic therapies, however newer molecules like upadacitinib were not included (2). This study aims to compare the relative efficacy of JAK inhibitors and TNF inhibitors for the treatment of active AS.
Methods: We conducted a Bayesian NMA of randomized clinical trials (RCTs) examining the relative efficacy of TNF inhibitors and JAK inhibitors in patients with active AS who had inadequate response or intolerance to NSAIDs. Systematic review was performed in PubMed, EMBASE and Cochrane databases until February 2020. Studies of IL-17 inhibitors were excluded after analysis showed inconsistency likely due to high placebo responses. NMA was conducted by Stata 16.0 software using odds ratio (OR) with 95% credible interval (CrI) to assess the clinical effectiveness. Surface Under Cumulative Ranking curve (SUCRA) was used to analyze the relative efficacy ranking of different treatments in terms of achievement of ≥20% in the Assessment of Spondyloarthritis International Society Criteria (ASAS20) at 12-16 weeks.
Results: We identified 19 RCTs that enrolled 3,654 patients with active AS. There were 120 pairwise comparisons including 20 direct comparisons of 16 interventions (figure 1). Compared with placebo, all the interventions showed an improvement in ASAS20 response rate, except for tofacitinib 2mg twice a day (bid) and tofacitinib 10mg bid dose groups (Table 1). Golimumab IV 2mg/kg showed the highest response rate (OR 7.74, 95% CrI 4.18- 14.34). The ranking probability based on the SUCRA indicated that golimumab IV 2mg/kg (SUCRA = 0.9), infliximab IV 5mg/kg (SUCRA = 0.8) and tofacitinib 5mg bid (SUCRA = 0.8) had the highest probability of achieving the best (optimal) outcome. We subjectively ranked the best therapies, based on SUCRA cut-offs, as optimal (SUCRA >0.8), good (SUCRA 0.5-0.7), or effective (SUCRA <0.4) (table 2). There were no differences in effectiveness between TNF inhibitors and certolizumab was the lowest ranked (SUCRA=0.2). These analyses were not substantially different if we applied an ASAS40 outcome, although there were fewer studies reporting ASAS40 results. The comparisons involving certolizumab, tofacitinib 10mg and upadacitinib were limited in the number of participants and, thus, may have been underpowered to detect statistical significance.
Conclusion: In patients with active AS, golimumab 2mg IV, infliximab 5mg IV and tofacitinib 5mg bid were most efficacious in achieving ASAS 20. JAK inhibitors seem to be an efficacious alternative in management of AS for which larger clinical trials are warranted.
References:
- Sinem S, et al. J Back Musculoskelet Rehabil. 31 (2018) 499–50
- Ungprasert P, et al. Clin Rheumatol. 2017 Jul;36(7):1569-1577
 Table 1. League table of pairwise comparisons for all treatments in the ASAS20 network meta-analysis.
Table 1. League table of pairwise comparisons for all treatments in the ASAS20 network meta-analysis.
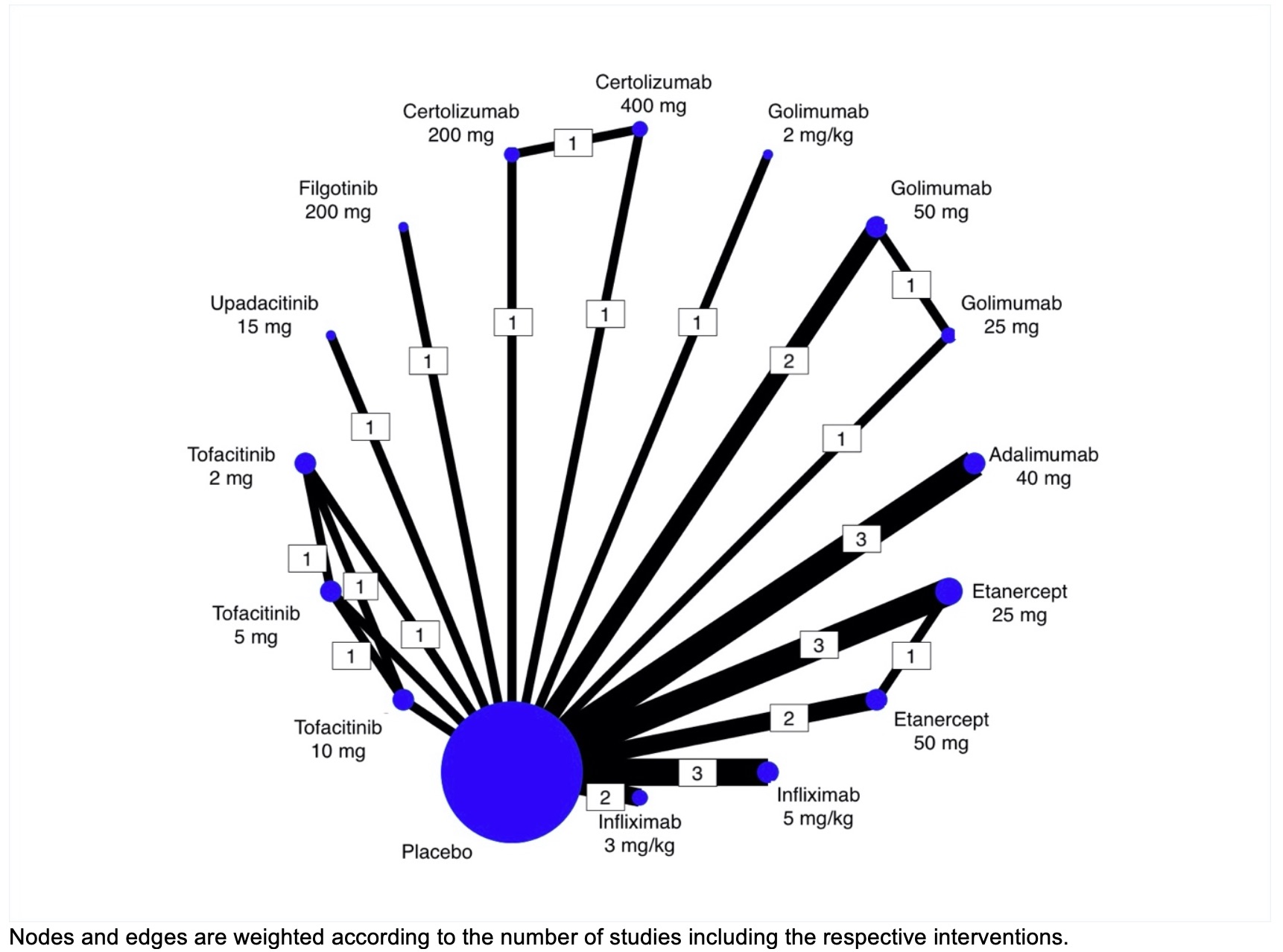 Figure 1. Network plot of the Active Ankylosing Spondylitis network (ASAS 20).
Figure 1. Network plot of the Active Ankylosing Spondylitis network (ASAS 20).
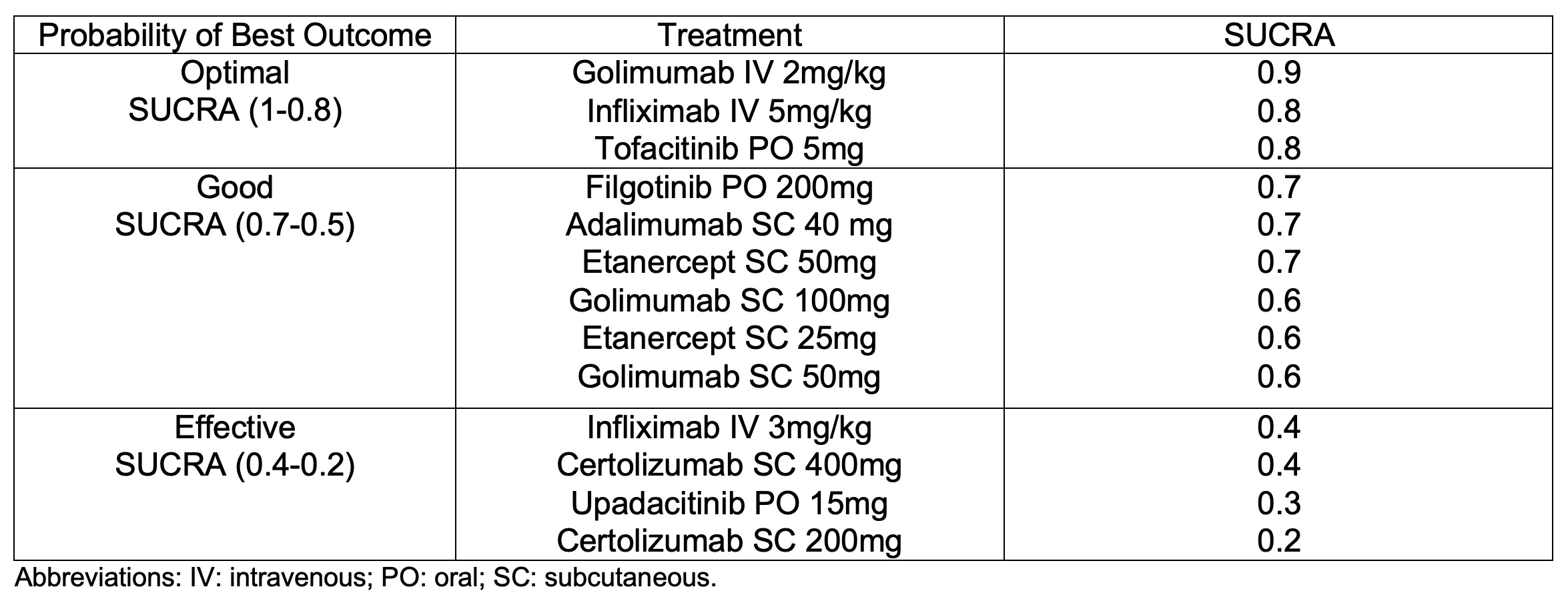 Table 2. The ranking probability of best outcome based on SUCRA.
Table 2. The ranking probability of best outcome based on SUCRA.
To cite this abstract in AMA style:
Castro A, Diaz J, Quiceno G, Cush J. Comparative Efficacy of Janus Kinase Inhibitors and TNF Inhibitors in Ankylosing Spondylitis: A Network Meta-Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparative-efficacy-of-janus-kinase-inhibitors-and-tnf-inhibitors-in-ankylosing-spondylitis-a-network-meta-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-efficacy-of-janus-kinase-inhibitors-and-tnf-inhibitors-in-ankylosing-spondylitis-a-network-meta-analysis/
