Session Information
Date: Sunday, November 12, 2023
Title: (0325–0344) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) and spondyloarthritis (SpA), including either Psoriatic Arthritis (PsA) and Ankylosing Spondylitis (AS), are some of the most commonly diagnosed autoimmune rheumatic diseases in rheumatologists’ routine clinical practice. Both can lead to joint destruction and substantial disability. Understanding patients’ health and functional status is crucial to provide personalized management strategies to optimize disease control and enhance the quality of life. We aimed to compare disease burden in patients with RA, PsA or AS by assessing Patient-Reported Outcome Measurement Information System (PROMIS) Physical Health, Global Mental Health, Physical Function and Fatigue 4a together with Visual Analogue Scale (VAS) Pain.
Methods: Data were obtained in the international COVID vaccination in autoimmune rheumatic diseases study second e-survey (COVAD study). Demographics, AIRD diagnosis, disease activity, PROMIS Global Physical health, PROMIS Global Mental Health, PROMIS Physical Function SF10 and PROMIS Fatigue 4a scores were extracted from the COVAD study database. For this study, we only included patients with self-reported RA or spondyloarthritis (either PsA or AS) undergoing active treatment with conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs) and/or biologic DMARDs, who answered all the survey questions. Active disease was defined as the patient’s perception of their disease as active in the four weeks before the first dose of COVID-19 vaccination. Analysis of Variance with Bartlett’s and Tukey’s test was used to compare continuous variables between groups.
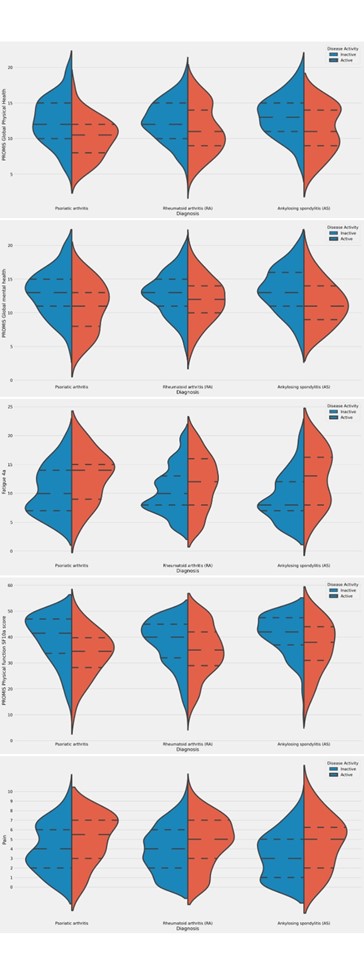
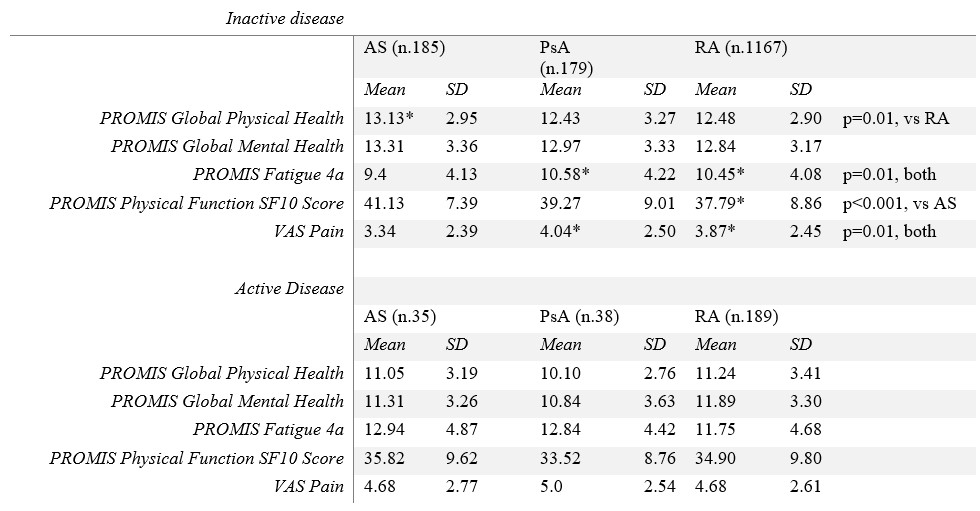
Results: From January to June 2022, 1907 patients with RA, female 87.62% (1671/1907), with mean age (±SD) 50.95 ± 13.67, 311 patients with PsA, female 67.20% (209/311), with a mean age of 50.42 ± 12.70, and 343 patients with AS, male 63.27% (217/343), with a mean age of 43.13 ± 12.75 years, responded to the COVAD e-survey. When assessed in those with active disease, neither physical health, global mental health, physical function, fatigue, nor pain were different among groups (Table 1, Figure 1). Patients with inactive AS had higher mean global physical health scores than RA patients (13.13 ± 2.93 VS RA 12.48 ± 2.90, p=0.01, Table 1). Nevertheless, those with inactive RA or PsA showed more severe fatigue (PsA 10.58 ± 2.22, RA 10.45 ± 4.08, vs AS 9.4 ± 4.13, p =0.01 for both). Patients with inactive RA also reported poorer physical function and more residual pain than those with AS (37.79 ± 8.86 VS 41.13 ± 7.79, p< 0.001; 3.87 ± 2.45 VS 3.34 ± 2.39, p=0.01, respectively). Similarly, residual pain was perceived as higher in patients with inactive PsA than those with AS (4.04 ± 2.50 VS 3.34 ± 2.39, p=0.01).
Conclusion: The burden of disease is approximately similar in patients with active RA, PsA or AS. However, patients with inactive RA and PsA report a considerably greater disease burden compared to those with AS.
To cite this abstract in AMA style:
Benavent D, Fornaro M, Iannone F, Cavagna L, Kuwana M, R N, Agarwal V, Day J, Joshi M, Saha S, Jagtap K, Katchamart W, Akarawatcharangura Goo P, Vaidya B, Velikova T, Sen P, Shinjo S, Tan A, Ziade N, Milchert M, Gracia-Ramos A, Caballero C, Chinoy H, Agarwal V, Aggarwal R, Gupta L, Venerito V. Comparative Disease Burden in Patients with Rheumatoid Arthritis, Psoriatic Arthritis or Ankylosing Spondylitis: Data from COVAD Patient-reported E-survey [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparative-disease-burden-in-patients-with-rheumatoid-arthritis-psoriatic-arthritis-or-ankylosing-spondylitis-data-from-covad-patient-reported-e-survey/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-disease-burden-in-patients-with-rheumatoid-arthritis-psoriatic-arthritis-or-ankylosing-spondylitis-data-from-covad-patient-reported-e-survey/