Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Knee osteoarthritis (OA) is an inflammatory disease, with a probable role for IL-1b. Calcium and urate crystals may promote OA by activating the NLRP3 inflammasome to produce IL-1b. Colchicine is a well-tolerated anti-inflammatory agent that inhibits the inflammasome and suppresses IL-1b. Studies examining the impact of colchicine on knee OA have yielded varying results, with some reporting pain relief, others improvement of inflammatory markers, and none assessing synovial effusions. We report the interim, blinded results of our ongoing colchicine trial for knee OA.
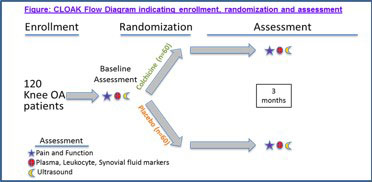
Methods: CLOAK is a randomized, double-blind, placebo-controlled trial of colchicine (once daily for 3 months) (Figure 1). We are enrolling subjects ≥ 40 years of age, with symptomatic knee OA, Kellgren-Lawrence grade 2 or 3 radiographs, and willingness to forego other anti-inflammatory therapy during the trial. The primary outcome is the change in knee pain by visual analog scale (VAS) after 3 months of treatment, comparing the colchicine and placebo groups. Secondary outcomes include pre to post treatment Knee Injury and Osteoarthritis Outcome Score (KOOS), mean doses of analgesics used, and changes in plasma and peripheral blood leukocyte inflammatory markers. Patients undergo knee ultrasound (US) pre- and post-treatment to assess synovitis and effusion. We aspirate synovial fluid when appropriate, and will analyze all available blood and synovial samples after study completion.
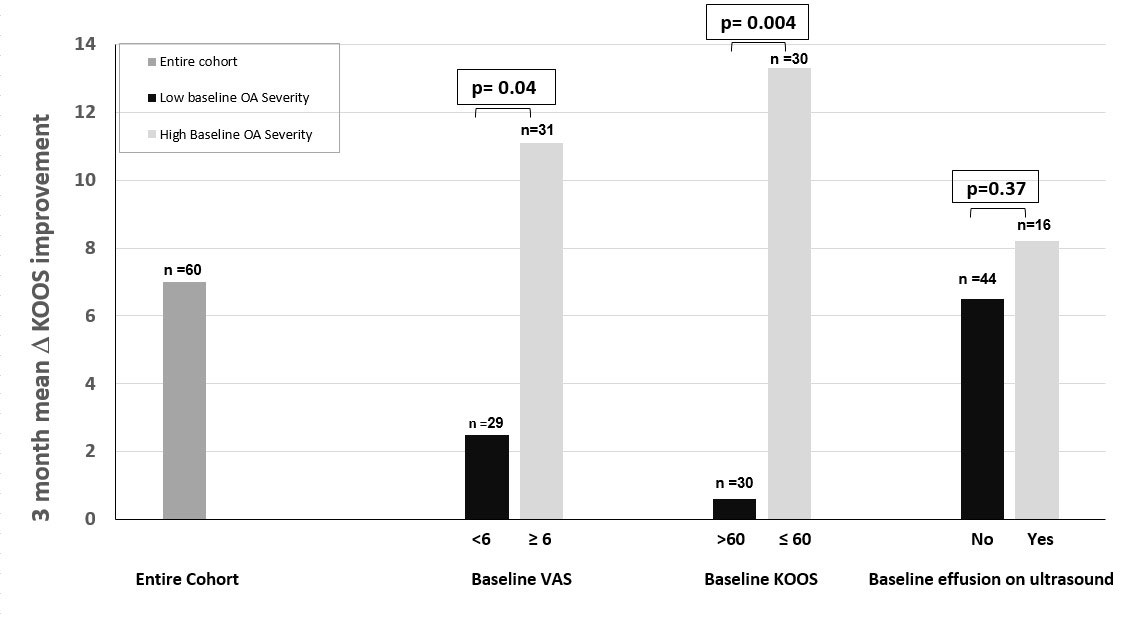
Results: To date, 715 potential subjects have been contacted, 82 screened, and 71 enrolled. Among 60 who have completed the study, 51.6% are male, 60% White, 30% Black, 3.3% Asian and 6.7% other, with mean BMI of 27.6 kg/m2 and age of 66.8 years. The mean VAS pain score among all completing participants (subjects and controls combined) improved by 0.98 units in the index knee, and mean KOOS scores improved for symptoms, pain, activities of daily living (ADL), sports activity, and quality of life (QOL). Overall 36 (60%) demonstrated VAS improvement (mean improvement 2.3) whereas 24 (40%) demonstrated no change or worsening. Overall, subjects whose VAS improved showed concordant improvement in the KOOS: mean symptoms by 10.5, pain by 12.4, ADL by 14.8, sports activity by 5.8 and QOL by 11.4 units. The subsets of patients with baseline VAS ≥6 and baseline KOOS ≤60 (i.e., more severe) showed significantly more 3-month KOOS pain improvement, even with the blinded inclusion of placebo (Figure 2). All underwent US at baseline and 3 months. Among 36 patients with VAS improvement over 3 months, 6 had baseline synovial effusions ≥4 mm (in longitudinal and transverse views) and 5 of these effusions were smaller on US post-treatment and one remained stable.
Conclusion: The results of this blinded analysis are consistent with a potential benefit of colchicine for pain, function and effusion in subjects not taking other anti-inflammatory agents. Enrollment is ongoing and the study will be unblinded and fully analyzed after completion.
To cite this abstract in AMA style:
Samuels J, Pillinger M, Toprover M, Krasnokutsky Samuels S, Patil A, Bomfim F, La Rocca Vieira R, Wei D, Catron S, coronel M, Kim A, Moussavi S. CoLchicine for Treatment of OsteoArthritis of the Knee (CLOAK)-A Double-blind, Placebo-controlled Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/colchicine-for-treatment-of-osteoarthritis-of-the-knee-cloak-a-double-blind-placebo-controlled-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/colchicine-for-treatment-of-osteoarthritis-of-the-knee-cloak-a-double-blind-placebo-controlled-trial/