Session Information
Date: Monday, November 14, 2022
Title: Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: To examine whether the use of colchicine and other gout medications is associated with the risk of COVID-19 infection, hospitalization, and subsequent outcomes in patients with gout.
Methods: We used the US National COVID Cohort Collaborative (N3C), the largest US cohort of COVID-19 cases and demographically matched controls, to identify patients with gout (International Classification of Diseases (ICD)-10 code, M1A or M10). We used multivariable logistic regression to assess the association between colchicine use and the odds of COVID-19 and COVID-19 outcomes (hospitalization, intensive care unit (ICU) admission, 30-day mortality, and World Health Organization (WHO) classification for COVID-19 severity etc.), compared to non-use of each medication, adjusted for demographics, medical comorbidities, smoking status, body mass index, region, and COVID-19 treatments.
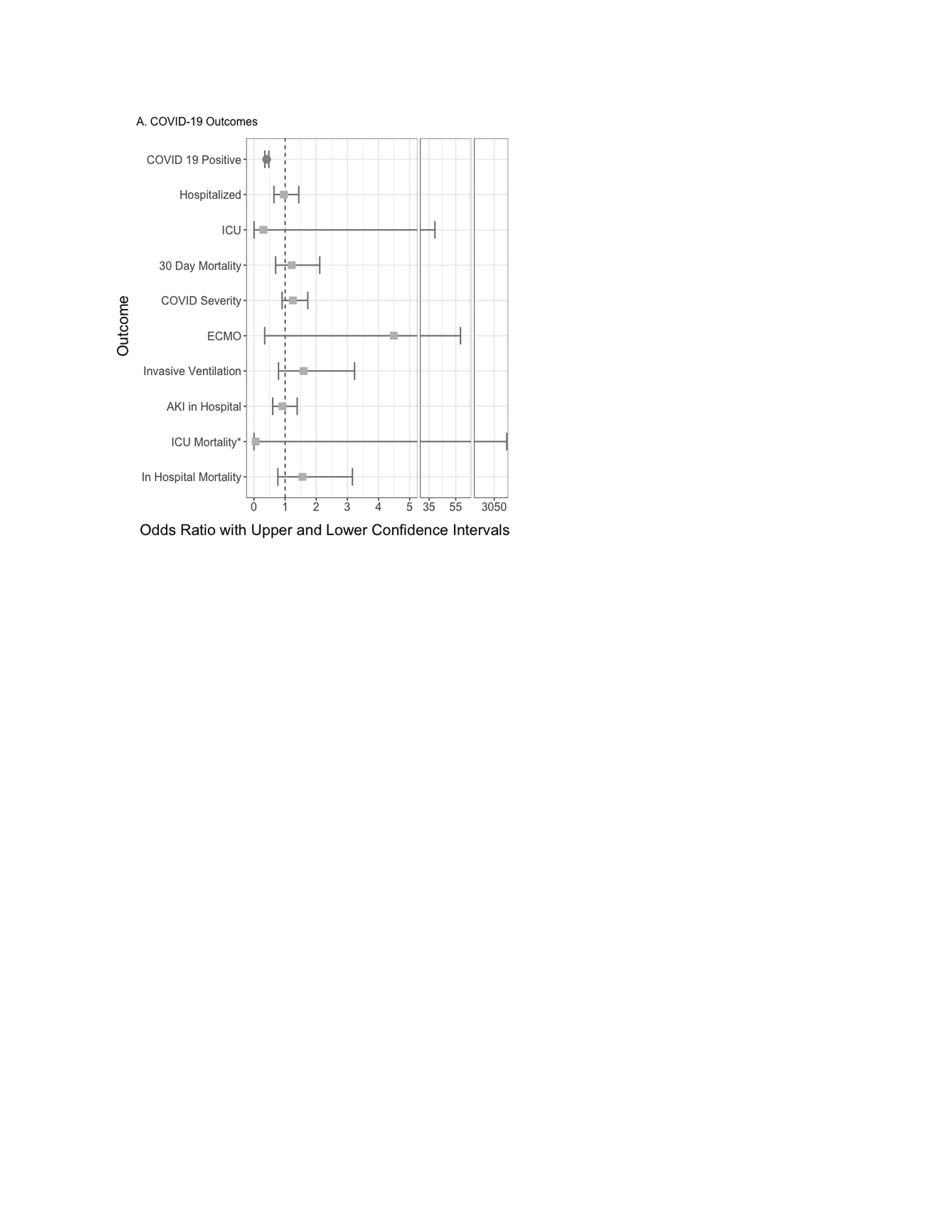
Results: The study cohort consisted of 147,060 people with gout; 54,727 (35%) had COVID-19 infection, 14,648 (26.8%) were hospitalized and 732 patients were exposed to colchicine within 30-days prior to their first COVID-19 diagnosis (1/1/2020 to 2/17/2022). Compared to non-use of the respective medication, colchicine use was associated with a decreased multivariable-adjusted odds ratio [aOR] (95% confidence interval [CI]) of COVID-19 infection, aOR 0.41 (95% CI, 0.35, 0.48) and allopurinol use was associated with an increased risk of COVID-19 infection, aOR 1.85 (95% CI, 1.58, 2.16); and anakinra use with higher 30-day mortality after COVID-19 infection, aOR 12.84 (1.76, 93.62). Colchicine use was not associated with significant differences in hospitalization, ICU admission, 30-day mortality, or COVID-19 severity (Figure). Results were confirmed in multiple sensitivity analyses.
Conclusion: Colchicine use in gout decreases and anakinra use increases the risk of COVID-19 infection. Patients with gout can consider the potential additional benefit/risk of continuing gout medications during the COVID-19 pandemic.
Figure 2 Legend: The figure shows the odds ratios and 95% confidence interval for the association between colchicine use and COVID_19-outcomes
AKI, acute kidney injury; CI: Confidence interval; ICU, intensive care unit; LOS: Length of stay
Hospitalized: adjusted for demographics, weight categories per BMI as normal vs. underweight, overweight, and obese, smoking status, US region, and modified Deyo-Charlson index
All other outcomes: adjusted for the above variables and all COVID_19 treatments
Circles (red) denote significant outcomes, orange squares denote non-significant outcomes.
*COVID outcomes did not have a sufficient sample size to calculate valid odds ratios.
To cite this abstract in AMA style:
singh J, Bergquist T, Madhira V, Anzalone A. Colchicine and Other Gout Medications and the Risk of COVID-19 Infection, Hospitalization, and Subsequent Outcomes in People with Gout [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/colchicine-and-other-gout-medications-and-the-risk-of-covid-19-infection-hospitalization-and-subsequent-outcomes-in-people-with-gout/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/colchicine-and-other-gout-medications-and-the-risk-of-covid-19-infection-hospitalization-and-subsequent-outcomes-in-people-with-gout/