Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Since the compound patent on the bio-originator of adalimumab expired, several adalimumab biosimilars (BS) have been introduced. Extensive research shows equivalence in effectiveness and safety, both for new patients and after transitioning from originator to biosimilar. However, different pharmaceutical properties might drive differences in tolerability. Patents on the modernised adalimumab bio-originator expired on different moments in time as effect of evergreening. This included reduced volume (0.4 ml instead of 0.8ml) and absence of citrate for the modernised bio-originator. This means that earlier adalimumab biosimilars differ in pharmaceutical properties from newer ones. Our aim is to describe the difference in outcomes between patients transitioning from the modernised bio-originator (0.4ml/no citrate) to BS1 (0.8ml/citrate) and from BS1 to BS2 (0.4ml/ no citrate) for all patients receiving treatment with adalimumab in the Sint Maartenskliniek in the Netherlands.
Methods: In this retrospective cohort study of patients with a clinical diagnosis of RA, PsA or axial SpA receiving adalimumab, two cohorts were identified. Patients who transitioned from the modernised bio-originator to BS1 (cohort 1) in 2021 and patients who transitioned from BS1 to BS2 (cohort 2) in 2023. Cohort entry was defined as the first time patients receive BS1 (cohort 1) or B2 (cohort 2) after the respective transition. The primary outcome was the 12 month drug survival of BS1 (after switching from bio-originator) and BS2 (after switching from BS1). Discontinuation of the biosimilar was counted as an event, except when the reason was pregnancy or remission. A multivariate cox regression analysis with a variance estimator was used, to adjust for confounding and account for data dependency because patients could be included in both cohorts. Secondarily, reasons for discontinuation were investigated separately for inefficacy and adverse events using hazard ratios (HR).
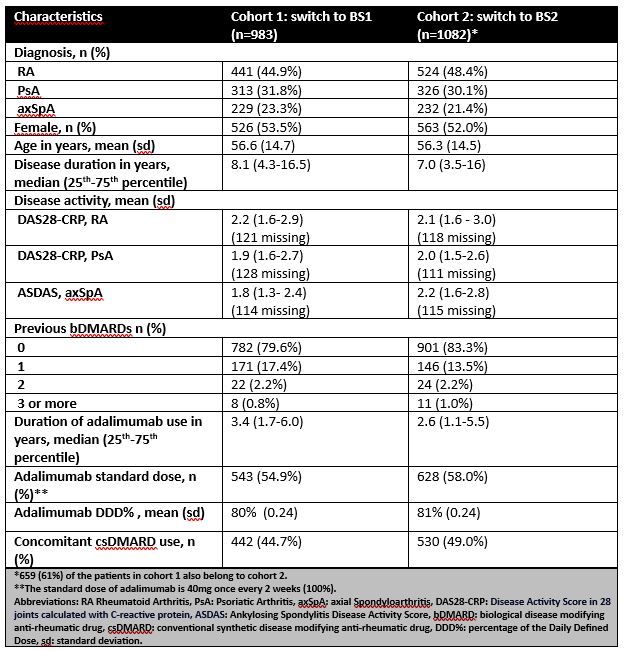
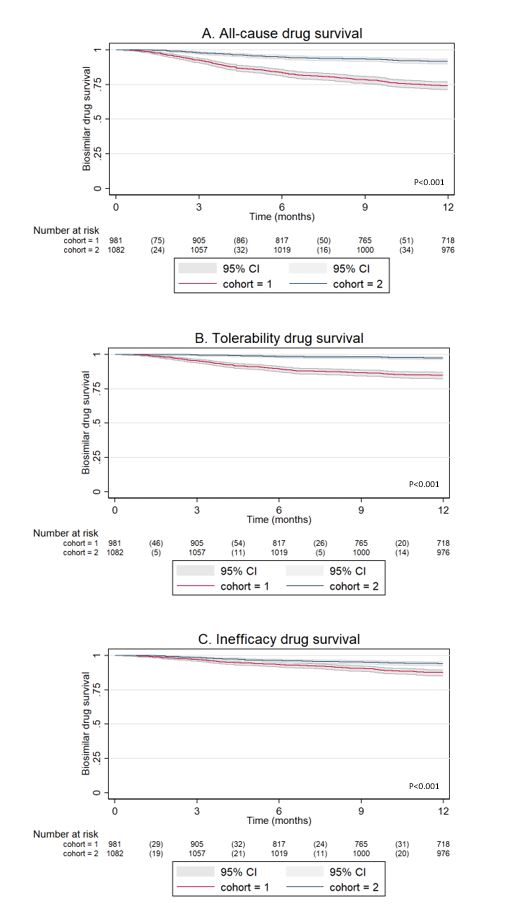
Results: In cohorts 1 and 2, 983 and 1082 patients transitioned from bio-originator to BS1 or from BS1 to BS2 respectively, with 659 patients in both cohorts (table 1). Drug survival rates at 12 months were 73% (95%CI: 70% to 76%) for cohort 1 and 90% (95%CI: 88% to 92%) for cohort 2 (p< 0.001) (figure 1A). The adjusted HR was 0.32 (95%CI:0.26-0.40) in favour of the newer BS2. The HR for discontinuation due to inefficacy between cohorts was 0.50 (95%CI 0.37-0.67) with better drug survival for cohort 2. For discontinuation due to adverse events the HR was 0.20 (95%CI 0.14-0.28) with better drug survival for cohort 2 (Figure 1B and 1C). The main tolerability issue was that BS1 injections were being experienced by patients as more painful than the bio-originator.
Conclusion: Adalimumab BS1 (0.8ml/citrate) has a significantly lower drug survival rate compared to BS2 (0.4ml/no citrate), and the difference is mainly driven by lower tolerability. These findings suggest that, when transitioning biosimilars, pharmaceutical differences can have an important impact on drug survival, and this should be taken into account.
Abbreviations: CI Confidence Interval.
To cite this abstract in AMA style:
Peeters A, Wientjes M, Müskens W, Ten Cate D, van den Bemt B, van Herwaarden N, den Broeder A. Cohort Study on Drug Survival and Tolerability of Adalimumab Biosimilar Transitioning: Pharmaceutical Properties Do Matter [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cohort-study-on-drug-survival-and-tolerability-of-adalimumab-biosimilar-transitioning-pharmaceutical-properties-do-matter/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cohort-study-on-drug-survival-and-tolerability-of-adalimumab-biosimilar-transitioning-pharmaceutical-properties-do-matter/