Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Rheumatoid arthritis (RA) patients are at increased cardiovascular (CV) risk, but traditional CV risk factors and available 10-year CV risk estimation models may not correctly intercept high-risk RA patients [1]. Phenotyping patients will likely undergo major cardiovascular events (MACE) is a clinical challenge, and unsupervised machine learning (uML) algorithms could be helpful in clinical practice. This study investigated whether an uML clustering analysis can determine the RA patient’s phenotype with different frequencies of MACE in a large RA cohort.
Methods: A cross-sectional multicentre cohort of RA patients fulfilling the 2010 ACR/EULAR classification criteria, free from previous CV events, was enrolled in January 2019 and longitudinally observed for new onset of MACE for 5 years. Clinical and demographic attributes were recorded, including traditional CV risk factors and the SCORE-2 algorithm [2], and MACE occurrence was tracked. Factor Analysis with Mixed Data (FAMD), a Principal Component Analysis generalization that can be deployed on large datasets with qualitative and quantitative variables, was implemented in a Python ver.3.9 environment using Prince ver. 0.7.1. Observations with missing data were ablated. The number of FAMD components to retain for clustering was assessed using bootstrapped eigenvalues distribution. Hierarchical Clustering on Principal Components was performed to define the number of clusters using Ward’s criterion and an Euclidian distance metric on the selected FAMD principal components. A supervised extreme gradient boosting (XGBoost) algorithm was run with cluster labels as the dependent variable to assess feature importance for cluster categorization. ANOVA and Chi-square were used to determine differences in the continuous and categorical variables distribution between clusters.
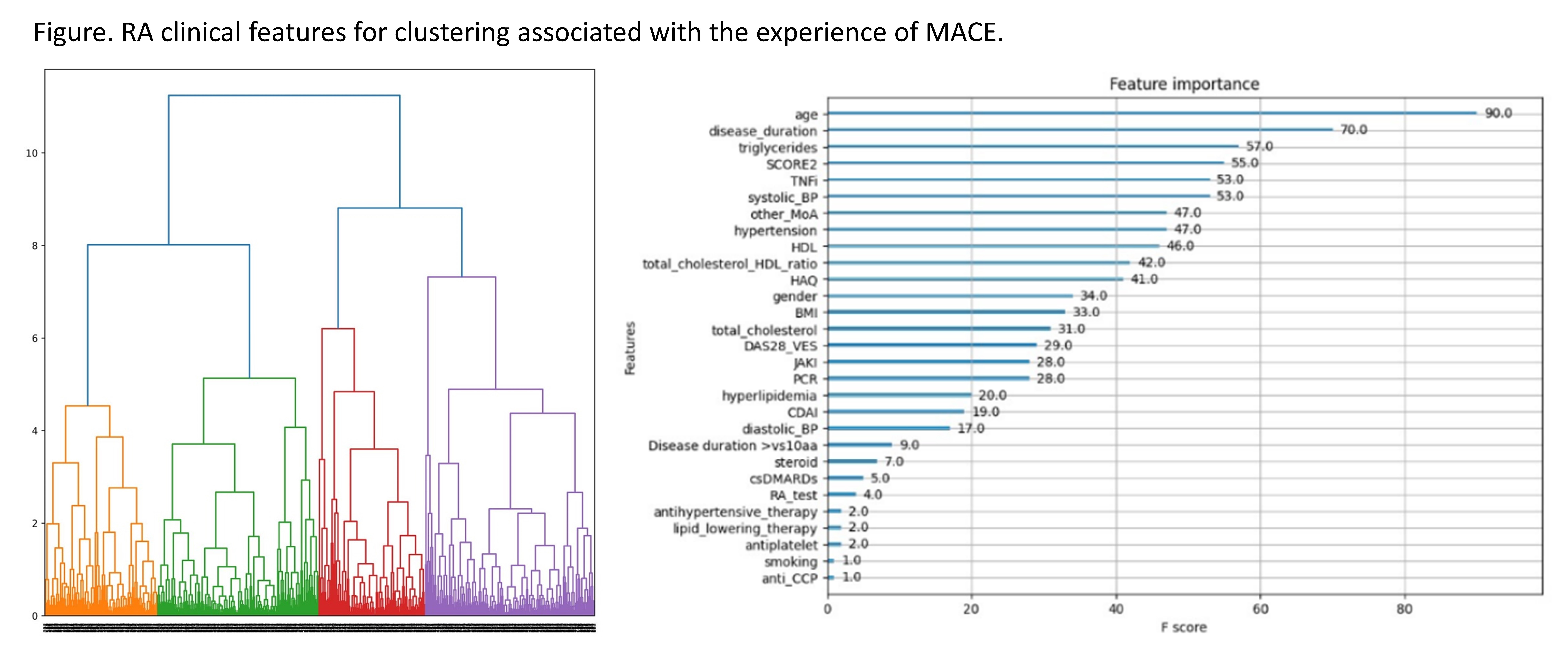
Results: Data from 951 RA patients (mean ±SD age 61.8±10.5 years, female 81.1%, and median (95%CI) disease duration of 120 (102-126) months) were analyzed. 18 MACE cases were recorded with an incidence rate of 4.73/1000 patient-years. Hierarchical clustering on two principal components retrieved n.4 clusters (see Figure). Age, disease duration, triglycerides serum level, and SCORE-2 were the most critical factors for clustering. Cluster 1: high HAQ, steroid user, and high total cholesterol values; Cluster 2: long disease duration, high systolic blood pressure, serum triglyceride level, and patients on lipid-lowering agents and TNFi; these patients presented with more MACE. Cluster 3: lower disease activity, BMI, diabetes, hypertension, and SCORE-2; Cluster 4: higher prevalence of male patients with disease < 10 years and on csDMARDs therapy; patients in these clusters did not experience any MACE.
Conclusion: Our uML-derived clustering identified specific clinical features of RA patients to consider together with the SCORE-2 chart. The portrayed disease phenotypes can corroborate CV risk models in recognizing those more likely to experience a MACE.
References:
1. Choy E et al. Rheumatology (Oxford) 2014; 53(12),2143-54
2. SCORE-2 Working Group and ESC CV risk collaboration. Eur Heart J 2021;42:2439-5
To cite this abstract in AMA style:
Cacciapaglia F, Venerito V, Erre G, Piga M, Manfredi A, Sakellariou G, Viapiana O, Gremese E, Bartoloni Bocci E, Spinelli F, Atzeni F. Clustering Analysis with Unsupervised Machine Learning Process to Phenotype the Cardiovascular Risk of Patients with Rheumatoid Arthritis Beyond the 10-year Prediction Algorithm [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clustering-analysis-with-unsupervised-machine-learning-process-to-phenotype-the-cardiovascular-risk-of-patients-with-rheumatoid-arthritis-beyond-the-10-year-prediction-algorithm/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clustering-analysis-with-unsupervised-machine-learning-process-to-phenotype-the-cardiovascular-risk-of-patients-with-rheumatoid-arthritis-beyond-the-10-year-prediction-algorithm/