Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA), an autoimmune polyarthritis characterized by ‘tumor-like’ proliferation of RA fibroblast-like synovial cells (RA-FLS), has characteristic symptoms such as “morning stiffness of joints” related to circadian rhythms. Furthermore, the concentrations of inflammatory cytokines such as TNF-α, IL-6, and IFN-γ in sera of RA patients is also known to peak at midnight, prior to arthritic symptoms in the morning. Circadian rhythms in individual cells are regulated by the expressions of clock genes (Bmal1, Clock, Per, and Cry). We previously reported that TNF-α increased the expression of Bmal1 in RA-FLS which induced osteochondral destruction via production of inflammatory mediators. It has been reported that BMAL1 binds to NF-κB/p65 in breast cancer cells, leading to promotes MMP-9 transcription (Wang et al. Cancer Cell Int, 2019, 19:182), however, it remains unclear how Bmal1 is involved in the pathogenesis of RA. In this study, we examined the function and the mechanism that Bmal1 contributed the productions of inflammatory mediators in RA-FLS under cytokine stimulations.
Methods: After transfected Bmal1/siRNA, RA-FLSs were stimulated with or without TNF-α (20 ng/ml), IL-1β (20 ng/ml) or IFN-γ (20 ng/ml) for 32 hours to examine the expressions of inflammatory mediators; CCL2, IL-6, MMP-3 by qPCR. Next, we investigated NF-κB/p65 activity and an interaction between BMAL1 and NF-κB/p65 by immunofluorescence and co-immunoprecipitation. Total protein was extracted from RA-FLS to analyze the expression of phospho-p65/total p65 by western blotting.
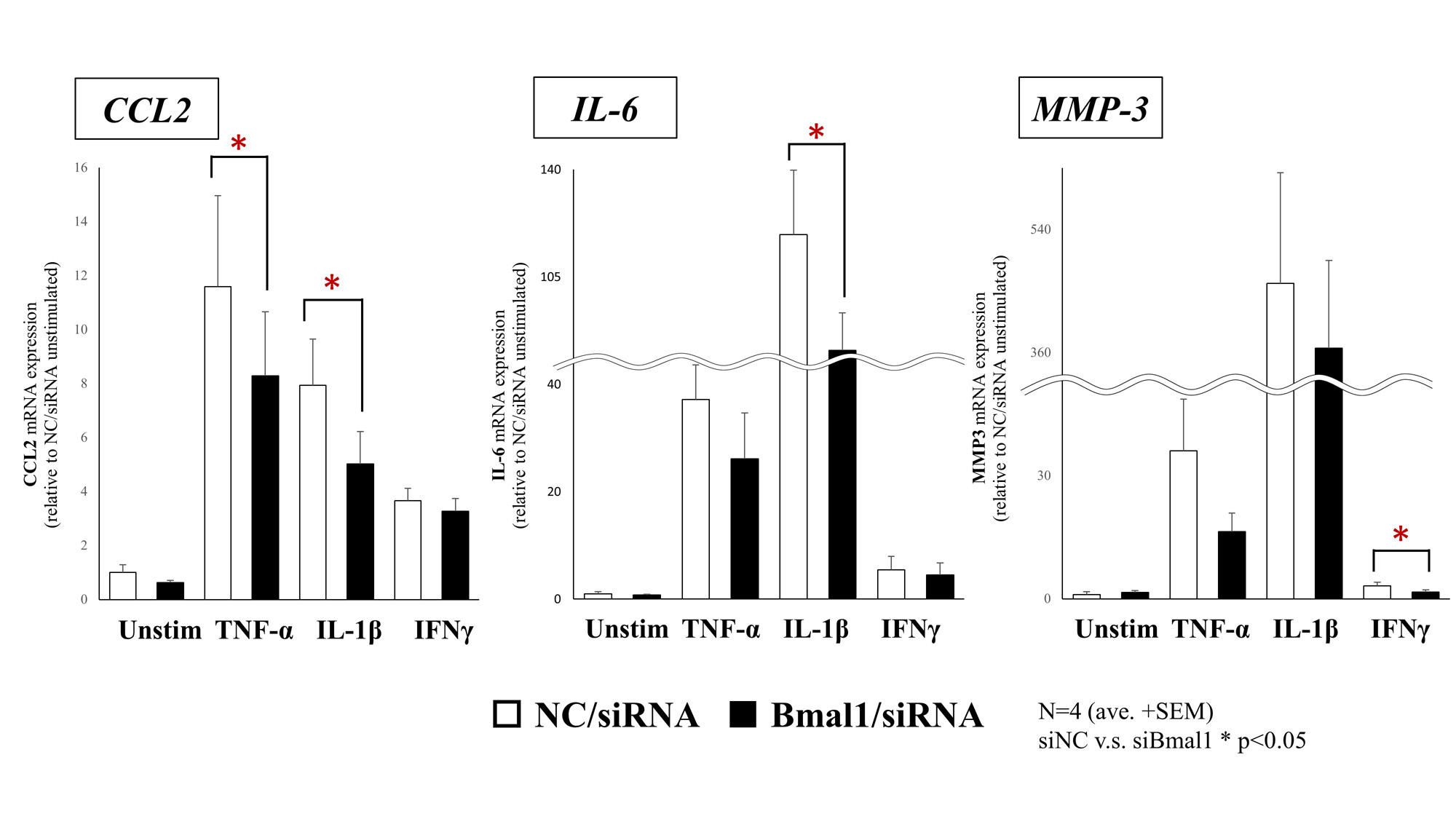
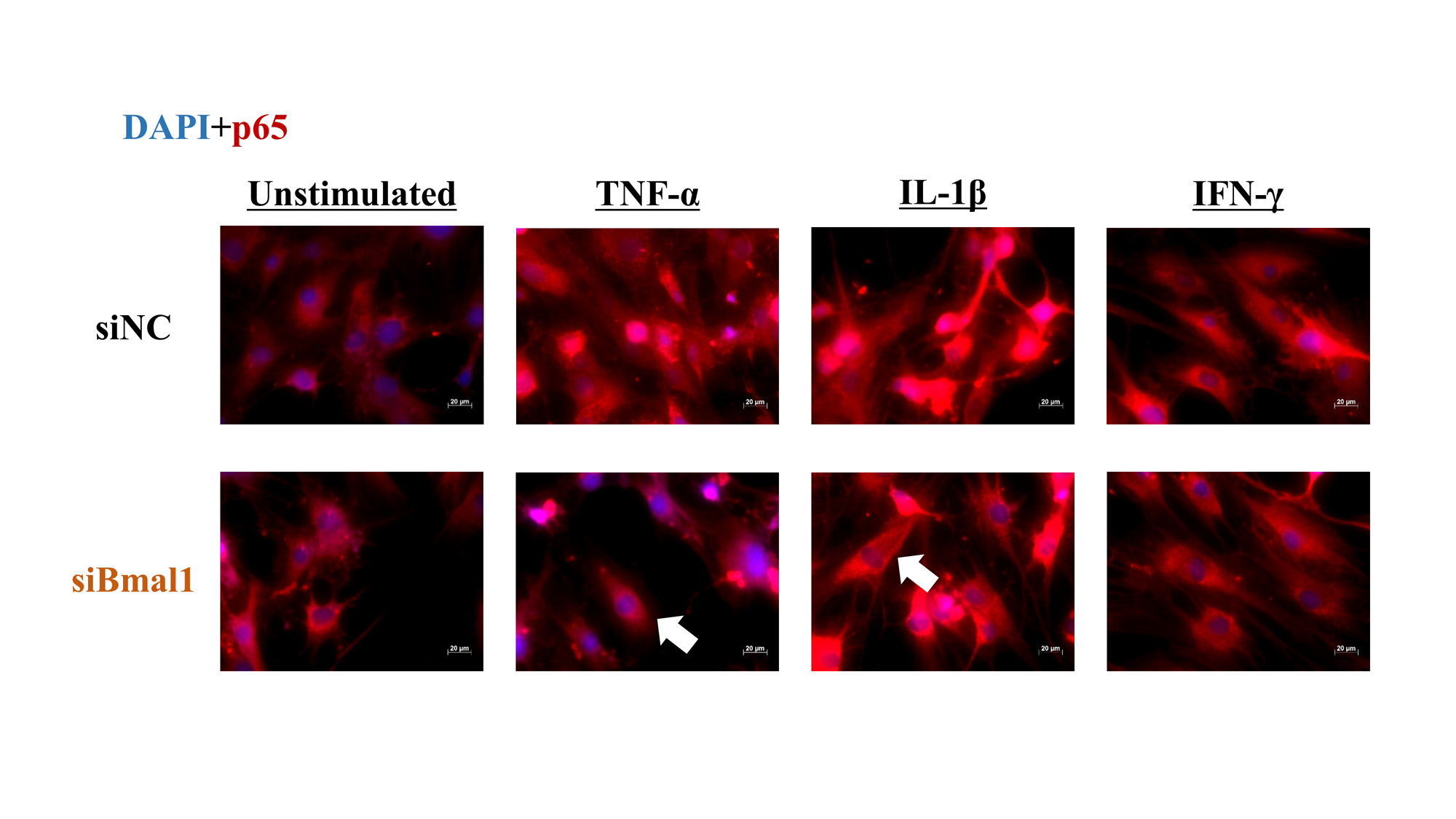
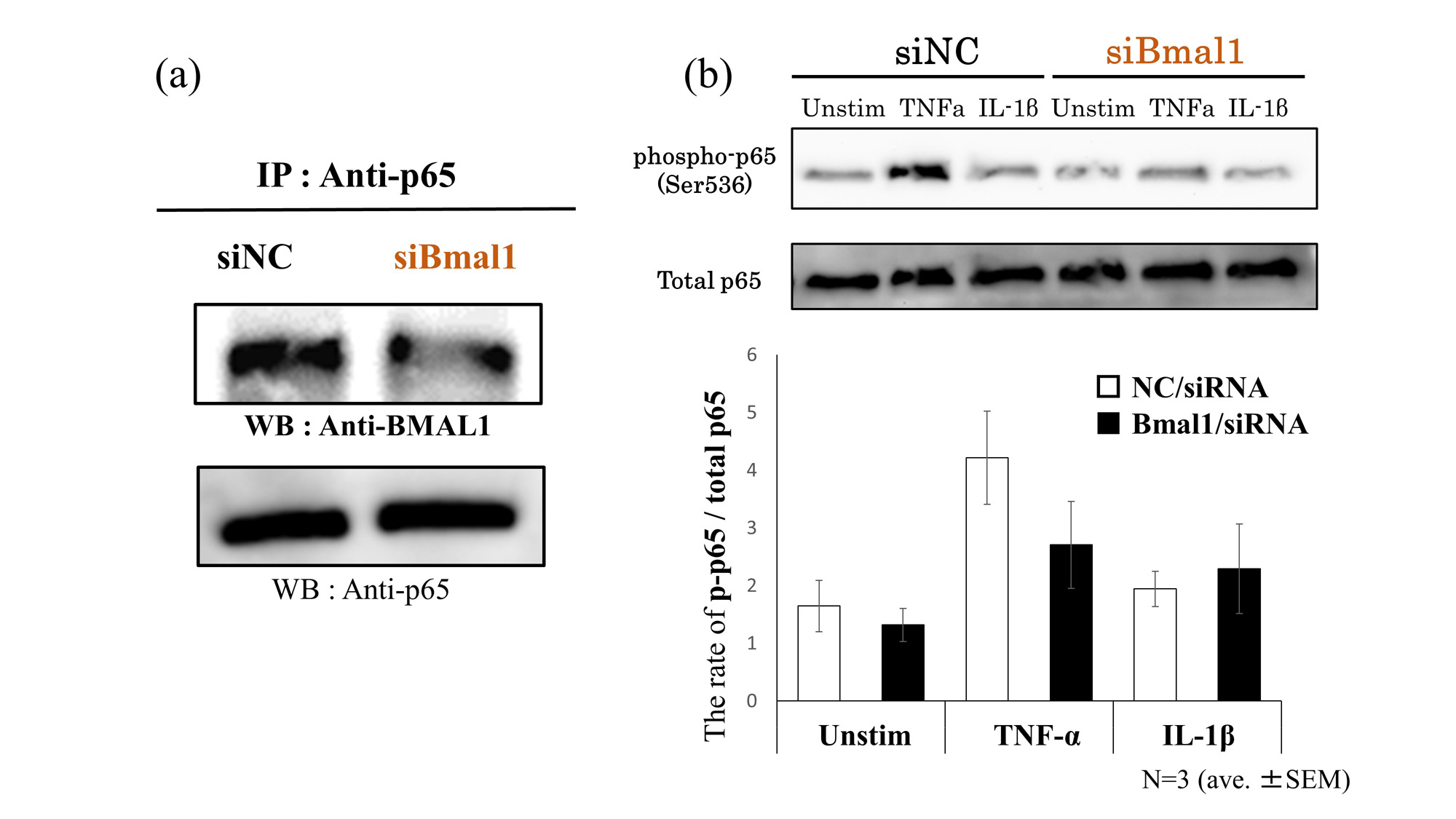
Results: Under stimulation of TNF-α and IL-1β, the mRNA expression of CCL2 were significantly suppressed by silencing Bmal1. Similarly, under stimulation of IL-1β and IFN-γ, silencing Bmal1 suppressed the mRNA expressions of IL-6 and MMP-3, respectively (Fig 1). Under fluorescence observations, NF-κB/p65 translocated into the nucleus following TNF-α and IL-1β stimulation though it appeared to be suppressed by silencing Bmal1. Stimulation with IFN-γ had no effect on p65 (Fig 2). Furthermore, BMAL1 was combined with p65 (Fig 3-a), and a phosphorylation of p65 was suppressed by silencing Bmal1 under stimulations with TNF-α (Fig 3-b).
Conclusion: The results indicated Bmal1 promoted the phosphorylation and nuclear translocation of p65 via forming the complex, subsequently contributing to the production of inflammatory mediators in RA-FLS, which might consequently induce osteochondral destruction in RA.
To cite this abstract in AMA style:
Tsukamoto H, Kaneshiro K, Yoshida K, Tateishi K, Terashima Y, Shibanuma N, Sakai Y, Hashiramoto A. Clock Gene Bmal1 Promotes Transcriptional Activity of NF-κB to Regulate Production of Inflammatory Mediators in RA-FLS [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clock-gene-bmal1-promotes-transcriptional-activity-of-nf-%ce%bab-to-regulate-production-of-inflammatory-mediators-in-ra-fls/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clock-gene-bmal1-promotes-transcriptional-activity-of-nf-%ce%bab-to-regulate-production-of-inflammatory-mediators-in-ra-fls/