Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite the progress in understanding the patient heterogeneity in systemic sclerosis (SSc) based on SSc-specific antibodies, better risk stratification is needed. SSc non-specific antibodies might represent surrogate markers to improve the stratification of SSc patients. Here, we aim to evaluate the prevalence of anti-Ro/SSA antibodies in the largest available cohort of established SSc patients and study their association with disease phenotype and clinical outcomes, focusing on lung involvement.
Methods: Patients from the EUSTAR database fulfilling the ACR 2013 classification criteria for SSc with available data on anti-Ro/SSA antibodies were included. Clinical characteristics of patients with or without anti-Ro/SSA antibodies were compared at baseline using t-test and chi-squared according to the distribution of the variable. The progression of lung fibrosis was defined (i) in patients with lung fibrosis (FVC%decline from baseline of ≥ 10% or an FVC%decline of 5-9% in association with aDLCO% decline of ≥15%)or (ii) by a decline of FVC >5% in patients with lung fibrosisor (iii) by the development of lung fibrosis de novo (on HRCT scan). Prognostic factors for lung fibrosis progression and death during the follow-up were tested by multivariate Cox proportional hazards regression. Covariates were selected according to literature evidence. Multiple imputation was used to impute missing data in these models.
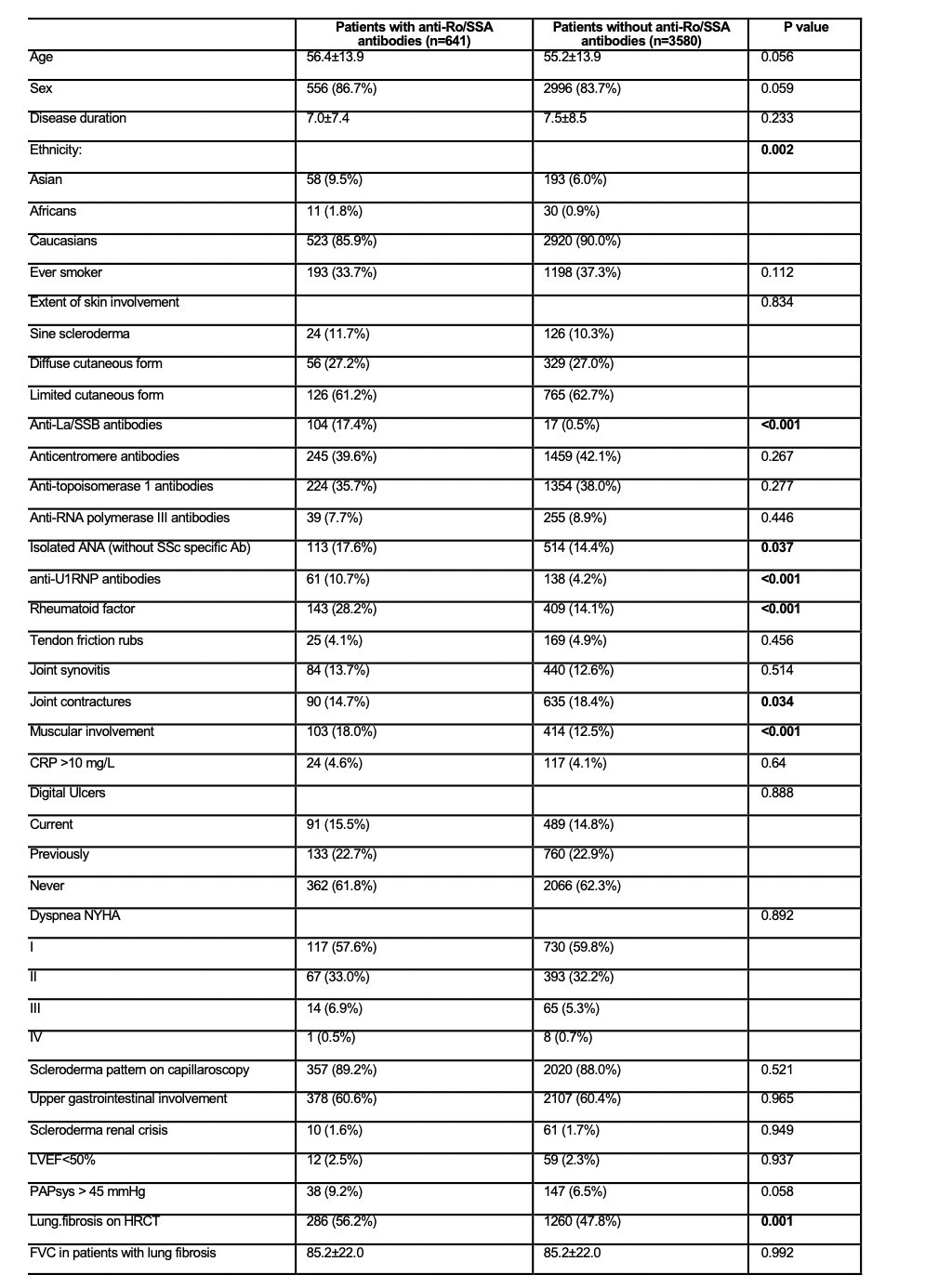
Results: Among the 4.421 patients fulfilling the inclusion criteria, 661 (15.2%) had positive anti-Ro/SSA antibodies. Anti-Ro/SSA antibodies were more frequently observed among Asians and Africans and less prevalent in Caucasians (Table 1). Anti-Ro/SSA antibodies were positively associated (p<0.001) with anti-SSB, anti-U1RNP antibodies, and rheumatoid factor. Patients with anti-Ro/SSA antibodies more frequently presented with muscular involvement (18% vs 12.5%, p<0.001), PAPs >45 mmHg on echocardiography (9.2% vs. 6.5%, p=0.058) and lung fibrosis on HRCT (56.2% vs 47.8%, p=0.001). Specifically, the percentage predicted of DLCO in patients with lung fibrosis was significantly lower in patients with anti-SSA antibodies (59.0±18.6% vs. 61.9±20.2, p=0.041). Over a median follow-up of 2.4 years [95CI: 2.2-2.9], anti-SSA antibodies did not predict lung fibrosis progression ((i) HR: 1.03 [0.8-1.33], (ii) HR: 1.05 [0.83-1.31] and (iii) HR: 0.98 [0.72-1.32] or death (HR: 1.27 [0.8-2]).
Conclusion: In the large EUSTAR cohort, anti-SSA antibodies are detected in 15% of SSc-patients and are associated with more severe lung involvement. These data support the inclusion of anti-SSA antibodies in clinical practice for better SSc-patient risk stratification.
To cite this abstract in AMA style:
Burja B, Boubaya M, Carreira P, Bergmann C, Ananieva L, Riemekasten G, Masado O, de Vries-Bouwstra J, Rosato E, Truchetet M, Del Papa N, Marcoccia A, ATZENI F, Schmeiser T, Vonk M, Del Galdo F, Distler O, Elhai M. Clinical Significance of Anti-Ro/SSA Antibodies in Patients with Systemic Sclerosis: A Study from the EUSTAR Database [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-significance-of-anti-ro-ssa-antibodies-in-patients-with-systemic-sclerosis-a-study-from-the-eustar-database/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-significance-of-anti-ro-ssa-antibodies-in-patients-with-systemic-sclerosis-a-study-from-the-eustar-database/