Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Itolizumab is a first-in-class, non-depleting, monoclonal antibody against the co-stimulatory receptor CD6 that blocks its interaction with ALCAM, to inhibit Teff cell activity and trafficking. It is being evaluated to treat immuno-inflammatory diseases where T cells play a central role, including active proliferative lupus nephritis (apLN). Here we present results from EQUALISE (Type B; NCT04128579), a Phase 1b study of itolizumab in subjects with apLN.
Methods: 17 adult subjects with apLN (ISN/RPS class III or IV with or without class V) were enrolled. All were treated with open-label itolizumab subcutaneously at 1.6 mg/kg Q2W for up to 13 doses in combination with mycophenolate mofetil (2-3 g/day) and systemic corticosteroids (methylprednisolone with rapid taper to prednisone < 10 mg/day by W10). Subjects were followed for 12 weeks after their last dose. Safety and efficacy measures were assessed.
Results: The median age of subjects was 34 years; 94% were female with 82% Asian; most subjects had ISN/RPS class IV+V disease (47%). Mean duration of LN was 5.4 years with Baseline mean 24 hour urine protein of 4.9 g and eGFR of 104 ml/min/1.73m2. Treatment was completed in 11 subjects with 4 discontinuing early (3 due to AEs and 1 due to physician decision) and 2 are still dosing.
88% of subjects experienced at least 1 adverse event (AE), most common were peripheral edema and lymphopenia. At least 1 low lymphocyte count was reported by 7 subjects (41%). Serious AEs occurred in 2 subjects (12%), including dehydration and COVID-19 infection, with none deemed related to study treatment.
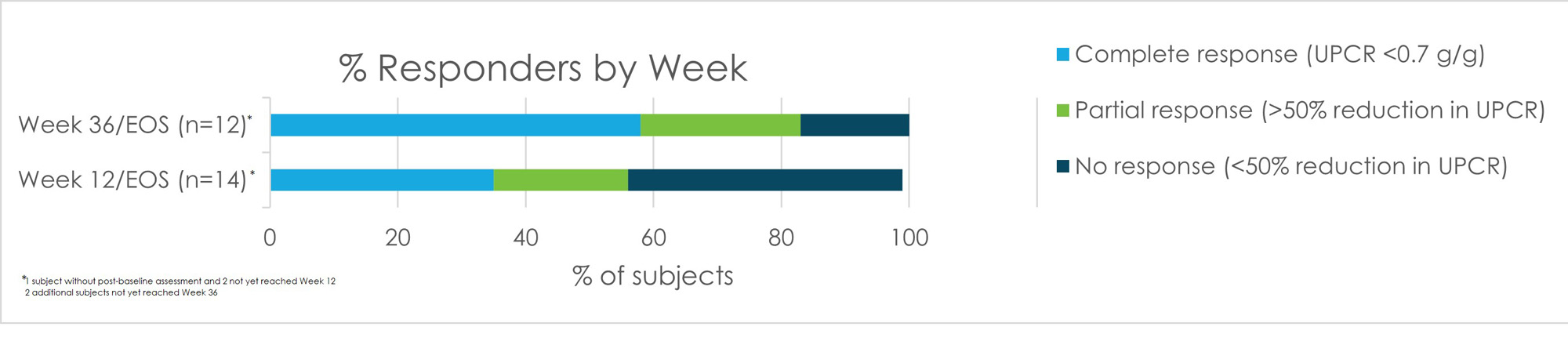
Based on the 14 subjects that completed/terminated the study and had a post-baseline measure, there was a median 72% reduction in spot urine protein creatinine ratio (UPCR), resulting in high partial and complete response (PR and CR) rates (FIGURE). At Week 12, the overall response rate was 57% (n=14), and at Week 36 (n=12) was 83%. 2 subjects are still dosing, and 2 are in the follow up period. These responses occurred as early as Week 4 on study with steroids tapered to a median of prednisone 5 mg by Week 12.
Conclusion: EQUALISE Type B demonstrates that subjects with proteinuric apLN had high CR and PR rates with rapid and deep reduction in UPCR when itolizumab was added to mycophenolate mofetil and corticosteroids. Further controlled studies are warranted in this population at high risk of disease progression and end stage kidney disease.
To cite this abstract in AMA style:
Kalunian K, Levin R, Parameswaran S, kopyt n, Connelly S, Sun E, Kim K, fung m, Rathi M. Clinical Safety and Efficacy Results from EQUALISE Type B: A Phase 1b Open-label Clinical Study of Itolizumab, a Novel anti-CD6 Therapy, in Subjects with Active Proliferative Lupus Nephritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-safety-and-efficacy-results-from-equalise-type-b-a-phase-1b-open-label-clinical-study-of-itolizumab-a-novel-anti-cd6-therapy-in-subjects-with-active-proliferative-lupus-nephritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-safety-and-efficacy-results-from-equalise-type-b-a-phase-1b-open-label-clinical-study-of-itolizumab-a-novel-anti-cd6-therapy-in-subjects-with-active-proliferative-lupus-nephritis/