Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA), a JAK1-selective inhibitor, demonstrated efficacy in the SELECT-BEYOND study in patients (pts) with moderate to severe rheumatoid arthritis (RA) on a stable dose of csDMARDs who had inadequate response (IR) or intolerance to bDMARDs,. In this analysis we evaluated clinical responses among pts receiving UPA and placebo (PBO) based on the number and mechanism of action (MOA) of prior bDMARDs.
Methods: 498 pts were randomized to UPA 15mg or UPA 30mg once daily (QD) or PBO for 12 weeks (wks), after which pts on PBO received UPA 15 or 30mg QD from Wk 12 onwards.1 Pts were subgrouped by the number and/or MOA of bDMARD(s) received prior to enrollment: 1) lack of efficacy (LoE) to ≥1 anti-TNF, 2) LoE to an anti-IL-6, and 3) the number of prior bDMARDs (1 vs 2 vs ≥3). ACR20/50/70 responses, DAS28-CRP low disease activity (LDA, ≤3.2), CDAI LDA (≤10), and CDAI remission (≤2.8) were evaluated at Wk 12. The frequency and percentage of treatment-emergent adverse events (TEAE) in each subgroup was assessed over the first 12 wks. Missing values of the efficacy endpoints were imputed using non-responder imputation (NRI). Nominal P-values are reported without multiplicity adjustment.
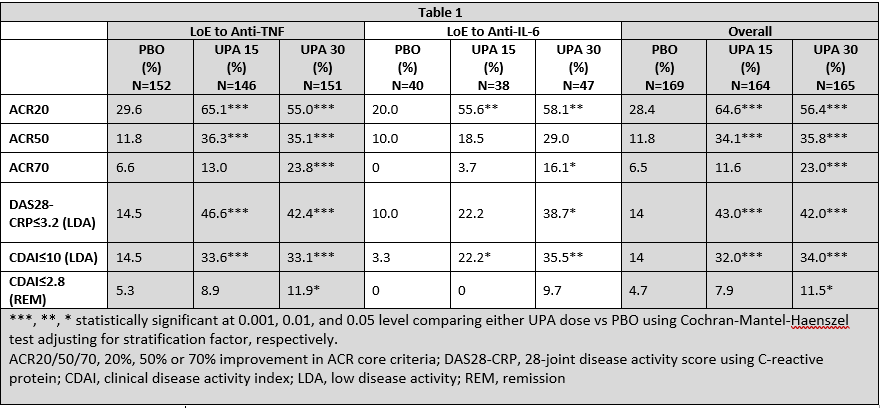
Results: Overall baseline disease duration was ~13 years. The majority of pts had LoE to ≥1 anti-TNF (449, 90%); 88 (18%) had LoE to an anti-IL-6; 235 (47%), 137(28%), and 125 (25%) had been treated with 1, 2, or ≥3 prior bDMARDs, respectively.1 At Wk 12, clinical responses were numerically, and often statistically, better for pts receiving either dose of UPA vs PBO, irrespective of their prior bDMARD exposure and the number of prior bDMARDs received. As most pts had LoE to ≥1 anti-TNF, responses in this group were comparable to the overall study population (Table 1). Pts with LoE to an anti-IL-6 receiving UPA 15 or 30 mg QD experienced improvements vs PBO, particularly in achieving LDA and remission, although responses in these pts were generally lower compared to the overall study population. As expected, there was a trend towards lower responses as the number of prior bDMARDs increased (Table 2). Responses at Wk 24 were generally consistent with those at Wk 12 (data not shown). TEAEs across the subgroups were consistent with the overall study population (data not shown).
Conclusion: At Wk 12, treatment with UPA at either 15 or 30 mg QD led to significantly better clinical responses vs PBO in this treatment-refractory population, including in pts with LoE to an anti-TNF or an anti-IL-6, and those who had IR/intolerance to 1, 2 or ≥3 prior bDMARDs, with consistent safety profiles as to the overall study population.
To cite this abstract in AMA style:
Weinblatt M, Thomson G, Chen K, Meerwein S, Schlacher C, Cush J. Clinical Responses in Patients with Inadequate Response to bDMARDs upon Treatment with Upadacitinib [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinical-responses-in-patients-with-inadequate-response-to-bdmards-upon-treatment-with-upadacitinib/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-responses-in-patients-with-inadequate-response-to-bdmards-upon-treatment-with-upadacitinib/