Session Information
Date: Sunday, November 13, 2016
Title: Metabolic and Crystal Arthropathies - Poster I: Clinical Practice
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Three randomized, double-blind, Phase III trials reported that greater proportions of patients treated with lesinurad 200 mg (LESU200) or 400 mg (LESU400), combined with the xanthine oxidase inhibitor (XOI) allopurinol (ALLO; CLEAR 1 and 2) or febuxostat (FBX; CRYSTAL), achieved serum uric acid (sUA) targets at 6 months versus xanthine oxidase inhibitor (XOI) alone. This analysis evaluated the impact of long-term treatment with lesinurad + XOI on tophus and flares for at least 1 year and up to 2 years.
Methods: Patients completing 12 months in the core CLEAR and CRYSTAL studies could enroll in respective uncontrolled extension studies (NCT01808131; NCT01808144). Patients randomized to LESU200 + XOI or LESU400 + XOI in the core studies who continued on combination therapy in the extension studies were analyzed. Efficacy endpoints included: (1) proportion of patients with complete resolution (CR) of ≥1 target tophus (ie, measurable tophus on hands/wrists and/or feet/ankles 5–20 mm in longest diameter), (2) percent reductions in the total area of all target tophi, and (3) proportion of patients experiencing a gout flare requiring treatment (GFRT).
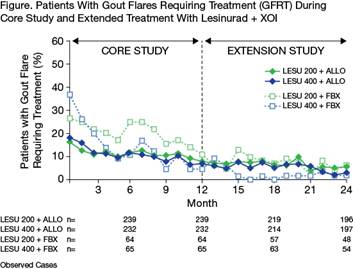
Results: A total of 239 (LESU200+ALLO) and 232 (LESU400+ALLO) patients continued in the CLEAR extension (n=32 and 33, respectively, with target tophi at baseline); 64 (LESU200+FBX) and 65 (LESU400+FBX) patients continued in the CRYSTAL extension. Proportion of patients with CR of ≥1 target tophus increased from end of core study (1 year) to 2 years: from 25.0% to 43.8% in LESU200+ALLO and 30.3% to 36.4% in LESU400+ALLO, and from 26.6% to 53.1% in LESU200+FBX and 35.4% to 58.5% in LESU400+FBX (LOCF). Percent reduction in the total area of all target tophi versus baseline changed from end of core study to 2 years: from 11.6% to 41.8% in LESU200+ALLO and 42.7% to 49.3% in LESU400+ALLO, and from 54.8% to 68.3% in LESU200+FBX and 58.6% to 72.4% in LESU400+FBX (LOCF). Proportion of patients with a GFRT per month decreased during continued combination treatment in both extension studies (Figure). The proportion of patients with a GFRT during Months 1, 12, and 24 were, respectively, 16.3%, 7.9%, and 5.6% in LESU200+ALLO; 18.1%, 6.9%, and 3.0% in LESU400+ALLO; 26.6%, 10.9%, and 6.3% in LESU200+FBX; and 36.9%, 4.6%, and 1.9% in LESU400+FBX. Extended treatment with LESU + XOI did not result in increased exposure-adjusted incidence rates of adverse events (AEs), AEs leading to discontinuation of lesinurad, serious AEs, or clinical laboratory abnormalities.
Conclusion: The CLEAR and CRYSTAL extension studies showed that patients treated with lesinurad + XOI for up to 2 years exhibited continued increases in the rate of complete resolution of tophi and reduction in tophus area, as well as decreased rates of gout flares.
To cite this abstract in AMA style:
Bardin T, Dalbeth N, Terkeltaub R, Storgard C, Fung M, Hu J, Perez-Ruiz F. Clinical Response of Tophus and Flares to Extended Use of Lesinurad in Combination with a Xanthine Oxidase Inhibitor in Patients with Gout [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/clinical-response-of-tophus-and-flares-to-extended-use-of-lesinurad-in-combination-with-a-xanthine-oxidase-inhibitor-in-patients-with-gout/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-response-of-tophus-and-flares-to-extended-use-of-lesinurad-in-combination-with-a-xanthine-oxidase-inhibitor-in-patients-with-gout/