Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: To analyse the clinical presentation, treatment response, and outcomes of arthritis induced by immune checkpoint inhibitors (ICIs) in patients with cancer.
Methods: Retrospective observational study conducted from January 2015 to December 2023 at four tertiary university hospitals.
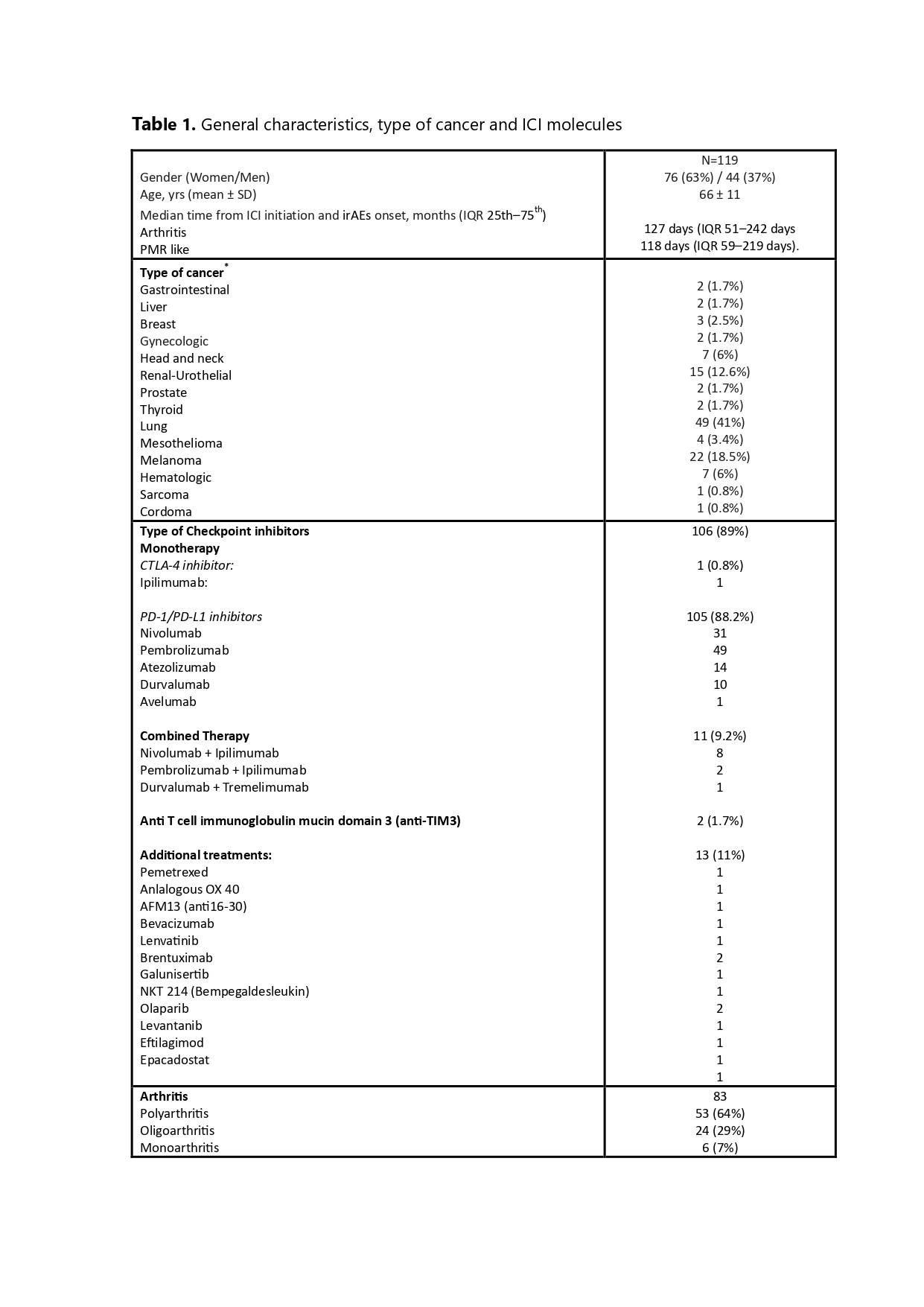
Results: We included 119 patients (63% male) with a mean age at diagnosis of 66 ± 11 years. Table 1 summarizes their general characteristics, cancer types, and ICI molecules.
Nine patients (7.5%) developed other rheumatic immune-related adverse events (irAEs), including sicca syndrome (9 cases), myositis (1 case), and sarcoidosis (1 case), while 41 (34.4%) experienced one or more non-rheumatic irAEs, usually occurring before or simultaneously with the rheumatic syndromes.
Of the 119 patients, 83 (70%) developed peripheral arthritis, and 36 (30%) exhibited a polymyalgia rheumatica (PMR)-like syndrome.
Among patients with peripheral arthritis, the median time from cancer diagnosis to ICI initiation was 6 months (IQR 25th–75th percentile: 1.8–14 months), and from ICI initiation to joint involvement was 127 days (IQR 51–242 days). Patients receiving combined therapy tended to develop symptoms earlier than those on monotherapy. Arthritis manifestations included polyarthritis (53 cases), oligoarthritis (24 cases), and monoarthritis (6 cases). The final diagnoses were undifferentiated arthritis in 53 (44.5%) patients, rheumatoid arthritis (RA)-like in 19 (16%), psoriatic arthritis-like in 8 (7%) and reactive arthritis-like in 3 (2.5%). Immunological markers showed positive ANA in 23.7% (14/59) and positive RF and ACPA in 3.3% (3/59).
Arthritis treatments included NSAIDs in 58% (48/83) of cases, GCs in 96% (80/83), HCQ in 44.5% (37/83), MTX in 12% (10/83), SSZ in 1.2% (1/83), and anti-TNF in 1.2% (1/83).
The median follow-up time for patients with arthritis was 9 months (IQR 3.4–16). At the last visit, 36% (30/83) achieved sustained drug-free remission (SDFR). ICI therapy was discontinued in 20 cases, while 10 patients continued their immunotherapy regimen without experiencing a relapse over a median follow-up of 9 months (IQR 6–23.09).
Among the 53 patients (64%) who were still under treatment for arthritis at the last visit, ICI therapy was ongoing in 49% (26/53) and had been discontinued in 51% (27/53). In the group of 27 patients where immunotherapy was discontinued, active arthritis persisted after a median follow-up of 8.20 months (IQR 3–17).
Among the 36 patients with PMR-like syndrome, the median time from cancer diagnosis to ICI initiation was 2 months (IQR 0.5–5.5 months), and from ICI initiation to PMR onset was 118 days (IQR 59–219 days). Besides GCs, 17% (6/36) were on steroid-sparing agents (MTX, leflunomide, or HCQ). The median follow-up was 12.4 months (IQR 5.2–21). At the last visit, ICIs were discontinued in 33.3% (12/36) of these patients, with 35% (11/36) achieving SDFR, while 69.5% (25/36) remained on treatment.
Conclusion: Forty-five percent of patients required a csDMARD / bDMARD. At the last follow-up, 65% of cases remained with active arthritis, either persistent or presenting intermittent flares. Persistence of artritis after the cessation of immunotherapy was observed in 58.7% of the cases.
To cite this abstract in AMA style:
Narvaez-García J, Llobell A, Ponce A, Gomez-Centeno A, Millán A, Corominas H, Nolla J, Gomez-Puerta J. Clinical Patterns and Long-term Outcomes of Arthritis Triggered by Immune Checkpoint Inhibitors. A Multicenter Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clinical-patterns-and-long-term-outcomes-of-arthritis-triggered-by-immune-checkpoint-inhibitors-a-multicenter-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-patterns-and-long-term-outcomes-of-arthritis-triggered-by-immune-checkpoint-inhibitors-a-multicenter-study/