Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
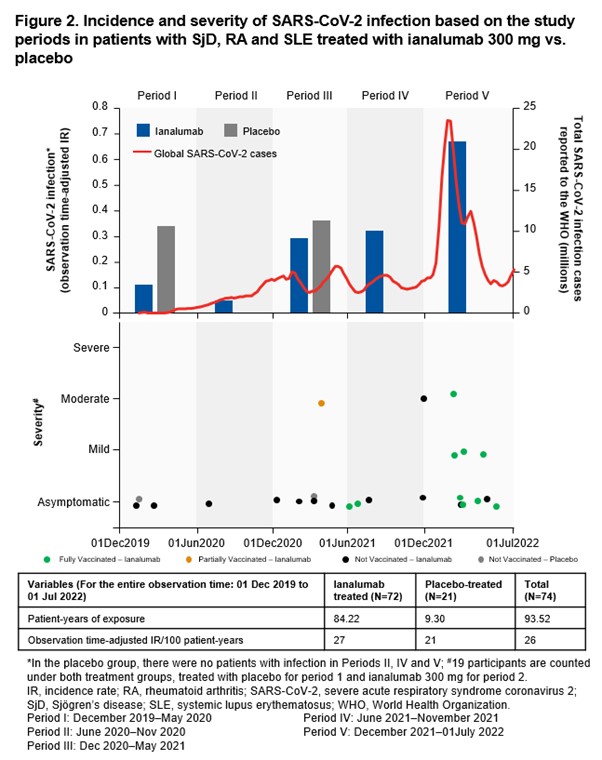
Background/Purpose: Ianalumab, a glycoengineered IgG1 mAb directed against B cell-activating factor (BAFF)-receptor (BAFF-R), targets B cells and their functions via dual mechanism: depletion of B cells through enhanced antibody-dependent cellular cytotoxicity and blockade of BAFF:BAFF-R–mediated signals.1 This study evaluates the incidence rate (IR) and severity of SARS-CoV-2 infections and serological response to SARS-CoV-2 infection and vaccination in patients with Sjögren’s disease (SjD), RA and SLE on ianalumab or placebo (SLE only).
Methods: In this retrospective analysis, patients with SjD (NCT02962895), RA (NCT03574545) and SLE (NCT03656562), who received at least one dose of ianalumab 300 mg or placebo on top of background therapies, between Dec 2019 – Jul 2022, and had anti-spike IgG, and/or anti-nucleocapsid IgG, and/or nucleocapsid antigen were included (Fig 1). Symptomatic SARS-CoV-2 infection was defined by treatment-emergent adverse events (AEs). Patients without reported SARS-CoV-2 AEs but with increased anti-nucleocapsid or nucleocapsid antigen levels were considered asymptomatic. Clinical outcomes included the incidence and severity of SARS-CoV 2–related AEs. Anti-spike IgG titers after exposure to SARS-CoV-2 antigen (virus/vaccine) were evaluated to assess the serological response (Fig 1).
Results: Overall, 123 and 28 patients who received ianalumab and placebo, respectively, were included. Of them, 74 (ianalumab: 72; placebo: 21) had anti-spike IgG, and/or anti-nucleocapsid IgG and/or nucleocapsid antigen data. The crude IRs of SARS-CoV-2 infection (not accounting for the different lengths of observation time/time at risk of SARS-CoV-2 infection) were 31.9% (23/72) and 9.5% (2/21), in the ianalumab and placebo groups, respectively.The observation time-adjusted IRs of SARS-CoV-2 infection were comparable between ianalumab and placebo groups (27 vs. 21 patients/100 patient-year). Most of the infected patients were asymptomatic in the ianalumab (17/23) and placebo (2/2) groups. Symptomatic infections in the ianalumab group (26.1%, 6/23) were mild to moderate in severity, and all patients fully recovered (Fig 2). Among the patients who received SARS-CoV-2 vaccine during this period and had available post-vaccination anti-spike IgG titer data (ianalumab: 10; placebo: 2), all mounted a serological response. Post-vaccination, anti-spike IgG titers were slightly lower in the ianalumab group during treatment, increasing during the follow up, reaching comparable levels to the placebo group after B cell recovery.
Conclusion: This analysis suggests that ianalumab treatment did not seem to increase the risk of severe SARS-CoV-2 infection in patients with SjD, RA or SLE. Both ianalumab and placebo groups mounted a serological response to SARS-CoV-2 vaccine, which improved during the follow-up even before B-cell recovery. Limitations include small sample size, imbalance in the patient number and observation time, varying risks, geographical regions, SARS-CoV-2 variants and vaccine availability.References:World Health Organization. WHO coronavirus (COVID-19) dashboard. Available at: COVID-19 cases | WHO COVID-19 dashboard. Accessed on December 18, 2024.
 *In patients positive for anti-nucleocapsid IgG or detectable nucleocapsid antigen or reported SARS-CoV-2–related AE; #In patients that had received SARS-CoV-2 vaccine as concomitant medicine; †anti-spike IgG, and/or anti-nucleocapsid IgG, and/or nucleocapsid antigen data.
*In patients positive for anti-nucleocapsid IgG or detectable nucleocapsid antigen or reported SARS-CoV-2–related AE; #In patients that had received SARS-CoV-2 vaccine as concomitant medicine; †anti-spike IgG, and/or anti-nucleocapsid IgG, and/or nucleocapsid antigen data.
AEs, adverse events; IgG, immunoglobulin G; PD, pharmacodynamic; RA, rheumatoid arthritis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SjD, Sjogren’s disease; SLE, systemic lupus erythematosus.
To cite this abstract in AMA style:
Devauchelle V, Ghanshani S, SIPS C, Hillenbrand R, Lau C, Hueber W, Bonal C, Oliver S. Clinical Outcomes and Response to SARS-Cov-2 Infection and Vaccination in Ianalumab‑Treated Patients with Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/clinical-outcomes-and-response-to-sars-cov-2-infection-and-vaccination-in-ianalumab%e2%80%91treated-patients-with-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-and-response-to-sars-cov-2-infection-and-vaccination-in-ianalumab%e2%80%91treated-patients-with-autoimmune-diseases/

.jpg)