Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The surge of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant Omicron infections has affected most Chinese residents at the end of 2022, including a number of patients with systemic autoimmune rheumatic diseases (SARDs). The aim of this study was to investigate clinical outcomes and the antibody level of the Omicron variant in SARDs patients after SARS-CoV-2 Omicron infection.
Methods: A total of 682 SARDs patients were surveyed. The type of SARDs, demographics, concurrent treatment, SARS-CoV-2 vaccination history, and clinical outcomes were recorded. Among these patients, we tested BA.5.2 and BF.7 Omicron variant IgG antibody level using enzyme-linked immunosorbent assay (ELISA) from the collected blood samples from 102 SARDs patients and 19 healthy controls (HCs).
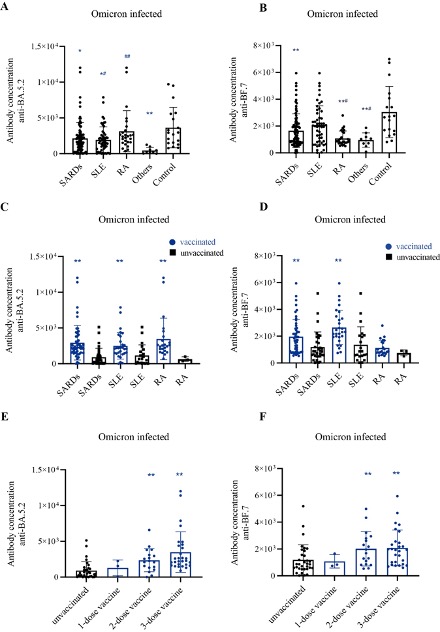
Results: 604 (88.6%) SARD patients were infected with SARS-CoV-2 during the pandemic, with the most common symptoms including fever (79.3%), cough (70.1%), malaise (39.1%), expectoration (31.0%), sore throat (28.7%), and ageusia (25.3%). The majority of patients (78.6%) had mild symptoms after SARS-CoV-2 Omicron infection. A total of 102 SARDs patients (mean age, 40.3 years, 89.2% female), including 60 SLE, 32 RA and 10 other SARDs, were tested for the antibodies against omicron variant IgG. We found that the BA.5.2 antibody level of infected SARDs patients was lower than that of HCs (P< 0.05), and a similar alteration was also observed in the antibody level of the BF.7 variant. Notably, the vaccinated SARDs group had notably higher levels of BA.5.2 and BF.7 antibodies than the unvaccinated group. Although the use of glucocorticoids (GC) does not affect the antibody levels, the level of BA.5.2 antibody was significantly decreased in SLE patients treated with bDMARDs compared with those treated with GCs and/or HCQ (P< 0.05). No significant difference in BF.7 antibody levels was found in SLE patients with different DMARDs use.
Conclusion: During the SARS-CoV-2 wave in China, in which the Omicron sublineages BA.5.2 and BF.7 were dominant, unvaccinated SARDs patients had lower antibody production, suggesting the need for valuable prevention and management strategies.
(A). Quantitative analysis of RBD antibody titers against the Omicron BA.5.2 variant calculated by ELISA. Compared with control, *P<0.05, ** P<0.01; compared with other SARDs group, #P<0.05, ## P<0.01. (B). Quantitative analysis of RBD antibody titers against the Omicron BF.7 variant calculated by ELISA. Compared with control, *P<0.05, ** P<0.01; compared with SLE group, #P<0.05. (C-D). Quantitative analysis of RBD antibody titers against the Omicron BA.5.2 (C) and BF.7 (D) variants calculated by ELISA between the vaccinated group and unvaccinated group, *P<0.05, ** P<0.01. (E-F). Quantitative analysis of RBD antibody titers against the Omicron BA.5.2 (E) and BF.7 (F) variants calculated by ELISA in SARDs patients with different vaccine doses, *P<0.05, ** P<0.01.
To cite this abstract in AMA style:
Chen Z, xiang n, Wang Q, Li Y, Jin H, Wang G, Li X, Jin T, Bao W. Clinical Outcome and Antibody Responses Following the Surge of SARS-CoV-2 Omicron Infection Among Patients with Systemic Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-outcome-and-antibody-responses-following-the-surge-of-sars-cov-2-omicron-infection-among-patients-with-systemic-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcome-and-antibody-responses-following-the-surge-of-sars-cov-2-omicron-infection-among-patients-with-systemic-autoimmune-diseases/