Session Information
Date: Sunday, October 26, 2025
Title: (0280–0305) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The six myositis core set measures (CSMs) are widely utilized to assess disease activity in idiopathic inflammatory myopathies (IIM). However, their association with how the patient feels and functions, and minimal thresholds for clinically meaningful improvement, remain undefined. We aimed to evaluate the validity, meaningfulness, and minimal clinical thresholds of change in CSMs in relation to symptoms, function, and quality of life in patients with IIM.
Methods: Adults with IIM were enrolled in a 6-month prospective observational study. Monthly data collected included CSMs (patient global assessments, Health Assessment Questionnaire [HAQ] and creatine kinase [CK]) and patient-reported outcome measures (PROMs) such as pain and fatigue (VAS), Short Form-36 (SF-36), and PROMIS-Physical Function (PROMIS-PF). Physician measures, including MMT-8 (scale 0-80), MDAAT, and physician global assessments, were gathered every three months. Baseline and longitudinal associations were evaluated using Spearman correlation and linear mixed models. Responsiveness, effect sizes, and clinically important differences (CID) were assessed using patient- and physician-reported change at 6 months as anchors.
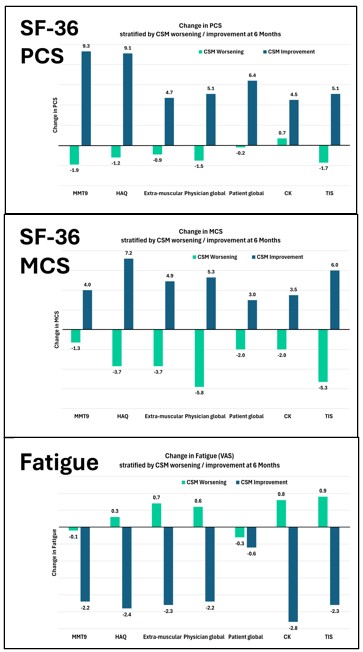
Results: Fifty patients (mean age 51.6 ± 14.9, 60% females) were enrolled, including 48% dermatomyositis, 12% polymyositis, 18% necrotizing myopathy, and 22% anti-synthetase syndrome. Most CSMs demonstrated strong baseline and longitudinal associations with key patient- and clinician-reported outcome measures, including pain, fatigue, physical function, and quality of life. CK showed weaker associations at baseline, but significant longitudinal correlations with most outcome measures (Table 1). In patients with improvement in absolute percentage change in the CSMs and TIS over 6 months, corresponding positive trends were observed in quality of life (PCS, MCS), fatigue (VAS), pain (VAS), and physical function (PROMIS-PF) (Figure 1). Specifically, greater improvements in patient and physician global assessments, disability (HAQ-DI), and muscle strength (MMT) were associated with incremental gains in quality of life and physical function measures, along with reductions in fatigue and pain. In patients who improved based on the patient global impression of change (PtGIC) and physician global impression of change (PhGIC) anchors, significant improvements were observed in physician global disease activity, MMT, HAQ-DI, CK, and extra-muscular disease activity, with moderate to large effect sizes. The CIDs according to PtGIC were 2.1 for physician global disease activity, 1.4 for extra-muscular disease activity, 1.7 for patient global disease activity, 4.2 for MMT, 0.5 for HAQ, and 1475 IU/L for CK levels. Similar results were observed for physician-reported improvement (Table 2).
Conclusion: Myositis CSMs showed strong validity and responsiveness, reflecting meaningful changes in patient symptoms, function, and quality of life, and supporting their utility in IIM clinical trials and practice. CIDs were defined for all CSMs, improving their interpretability and application in clinical trials and routine care.
Longitudinal correlations between core set measures (CSMs) and outcome measures using linear mixed models adjusted for age, sex, and race. Most CSMs showed strong associations with patient-reported outcomes and other clinical metrics, supporting their validity in tracking disease activity in IIM.
Changes in patient-reported outcomes (PCS, MCS, and Fatigue VAS) stratified by levels of improvement in CSMs and TIS over 6 months. Greater improvements in core measures were associated with meaningful gains in quality of life and symptom burden, as perceived by patients.
Six-month changes, effect sizes, and clinically important differences (CIDs) of CSMs in groups defined by patient- and physician-reported improvement. These values establish thresholds for meaningful clinical change and highlight the responsiveness of individual CSMs and TIS.
To cite this abstract in AMA style:
Keret S, Lomanto Silva R, Choudhuri I, Gkiaouraki E, Chandra T, Pongtarakulpanit N, Sriram S, Bhowmick N, Kothari V, Sreerama Reddy K, Alhassan E, Aggarwal A, Almackenzie M, Moghadam-Kia S, Ascherman D, V. Oddis C, Aggarwal R. Clinical Meaningfulness and Improvement Thresholds of Myositis Core Set Measures: Association with Patient-Reported Outcomes [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/clinical-meaningfulness-and-improvement-thresholds-of-myositis-core-set-measures-association-with-patient-reported-outcomes/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-meaningfulness-and-improvement-thresholds-of-myositis-core-set-measures-association-with-patient-reported-outcomes/

.jpg)
.jpg)