Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: SLE is an autoimmune disease with diverse manifestations and progression patterns that, via disease activity and effects of standard therapy, imposes a significant burden on patient health-related quality of life (HRQoL) and long-term survival.1,2 The aim of the international SLE Prospective Observational Cohort Study (SPOCS) was to describe the comprehensive patient journey with SLE; here, we focus on patients in the United States (US).3
Methods: SPOCS (NCT03189875) followed adults with moderate to severe SLE at biannual clinic visits for up to 3 years.3 In this analysis, patients were adults in the US with SLE (SLICC/ACR criteria), modified SLEDAI-2K score ≥4 (lupus headache and urine/laboratory results, including immunologic measures, excluded) or SLEDAI-2K score ≥6, positive ANA or dsDNA status, and minimum 6-month systemic treatment for active SLE; patients with active severe LN were excluded.3 Longitudinal data on disease activity (SLEDAI-2K), remission (Definition of Remission in SLE, DORIS4), Lupus Low Disease Activity State4 (LLDAS), organ damage (SLICC/ACR Damage Index [SDI]), medication use, and HRQoL (Patient Health Questionnaire–8 [PHQ–8], Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT–Fatigue]) were analyzed descriptively.
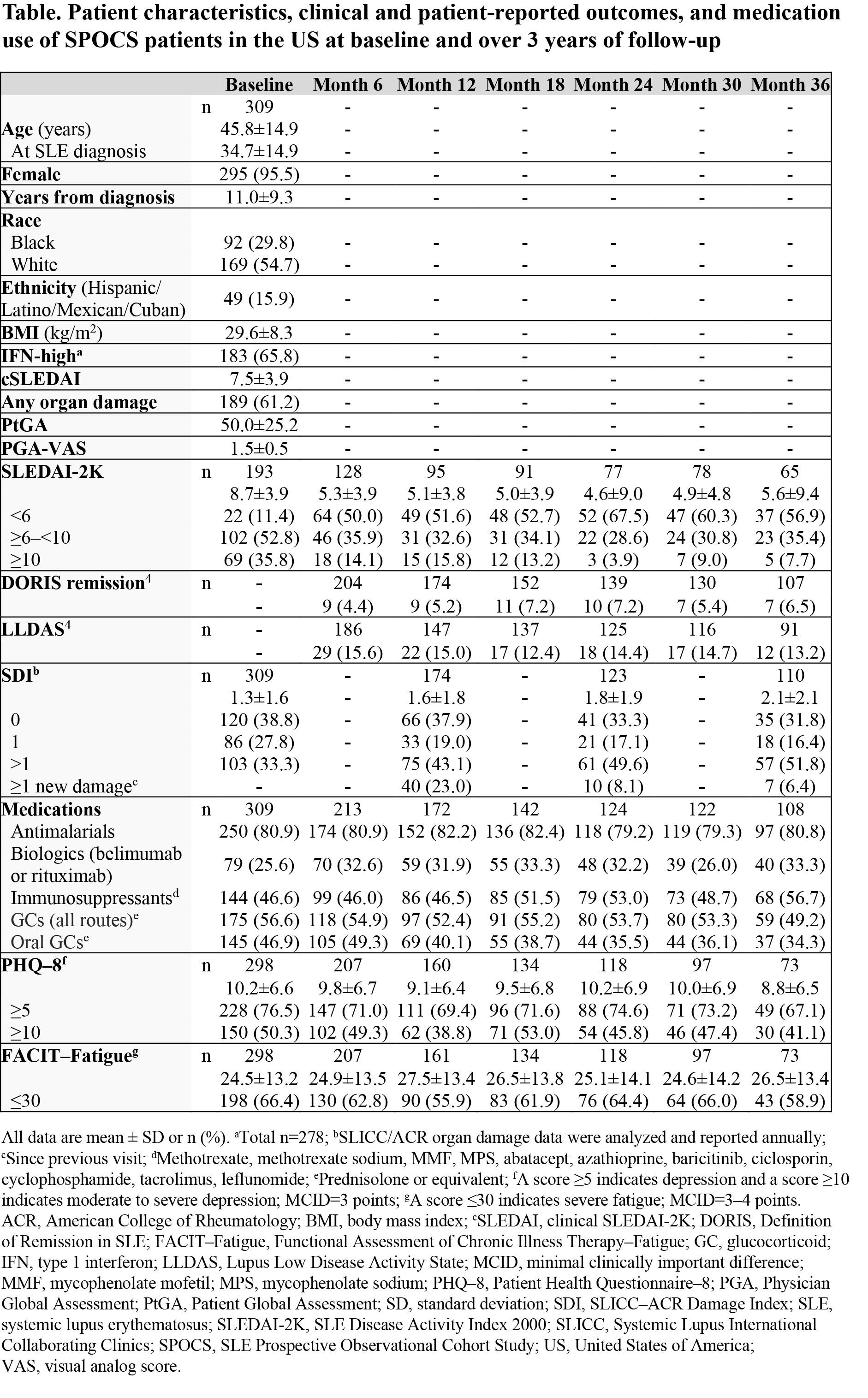
Results: Of 826 patients enrolled in SPOCS, 309 (37.4%) were in the US. Most of these patients were female, White, with a mean (SD) 11.0 (9.3) years since SLE diagnosis at baseline (Table). Glucocorticoids were used by 56.6% (175/309) of patients at baseline.
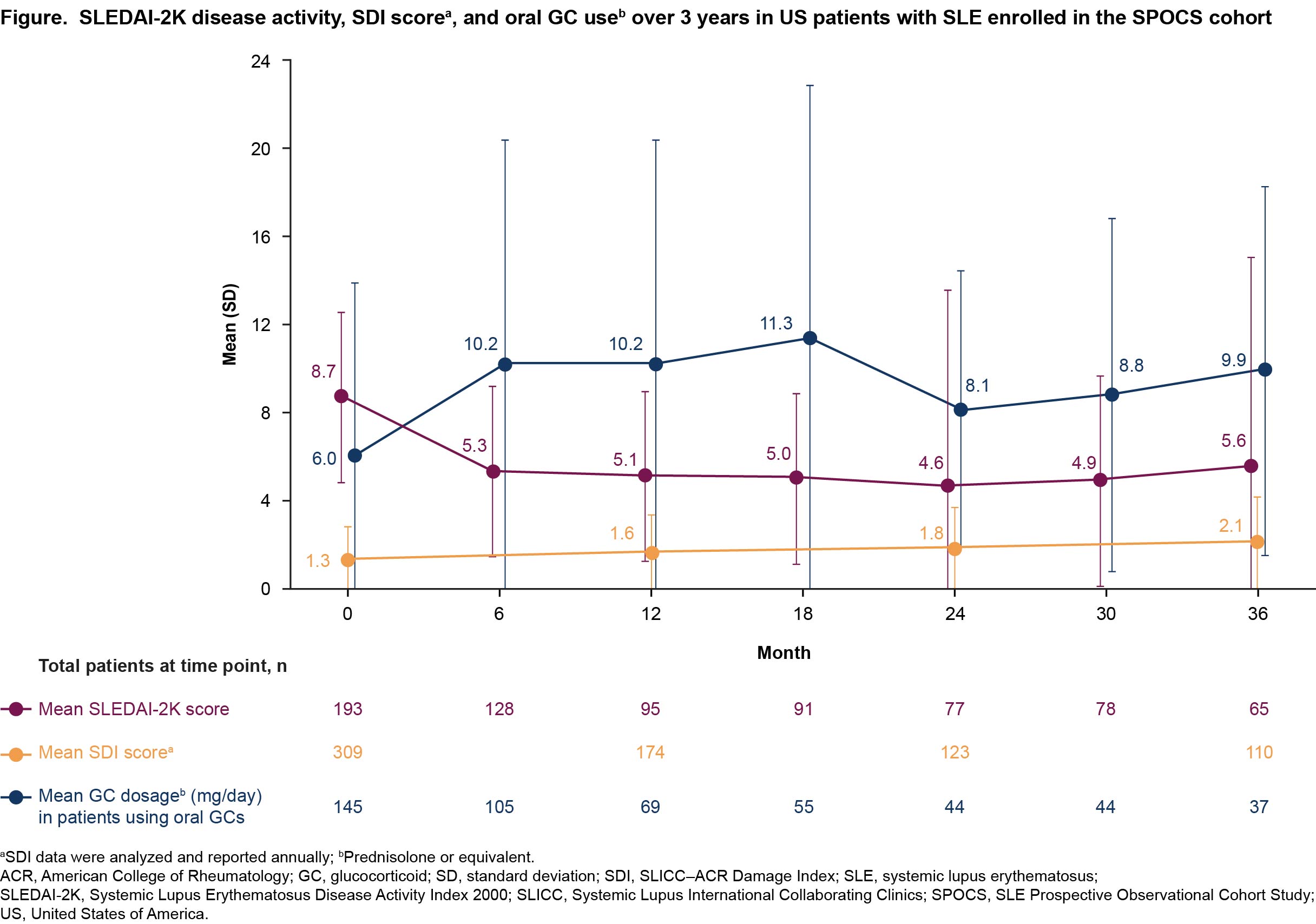
The mean (SD) baseline SLEDAI-2K score decreased from 8.7 (3.9) to 5.3 (3.9) at Month 6, then remained stable over time (Figure). Over 36 months, < 10% of patients achieved DORIS remission and < 20% of patients achieved LLDAS at any given study visit (Table). The mean (SD) SDI score increased annually from 1.3 (1.6) at baseline. At Month 12, 23.0% (40/174) of patients had new organ damage or deterioration (ie, any increase in SDI). Among patients receiving glucocorticoids, mean (SD) prednisolone or equivalent dosage increased from 6.0 (7.9) mg/day at baseline to >7.5 mg/day for the rest of the study. The proportion of patients reporting depression (PHQ–8 ≥5) was high at each study visit (baseline: 76.5% [228/298]; Month 36: 67.1% [49/73]), and over half of patients at a given study visit reported severe fatigue (FACIT–Fatigue ≤30; baseline: 66.4% [198/298]; Month 36: 58.9% [43/73]).
Conclusion: Although disease activity improved from Months 0–6, DORIS remission and LLDAS attainment rates were consistently low during the 3-year SPOCS study. Organ damage increased over time, and HRQoL was impacted by high rates of depression and severe fatigue. Overall, there is an unmet need for improved treatment outcomes among patients with SLE in the US.
References:
1. Fanouriakis A, et al. Ann Rheum Dis. 2021;80(1):14–25.
2. Bruce IN, et al. Ann Rheum Dis. 2015;74(9):1706–13.
3. Arnaud L, et al. Lupus Sci Med. 2023;10(2):e001032.
4. Golder V, et al. Rheumatol. 2020;59(Suppl 5):v19–v28.
To cite this abstract in AMA style:
Furie R, Binka M, Atefi G, Chen S, Ding B. Clinical and Patient-reported Outcomes in Systemic Lupus Erythematosus: An Analysis of the SLE Prospective Observational Cohort Study (SPOCS) US Data [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clinical-and-patient-reported-outcomes-in-systemic-lupus-erythematosus-an-analysis-of-the-sle-prospective-observational-cohort-study-spocs-us-data/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-and-patient-reported-outcomes-in-systemic-lupus-erythematosus-an-analysis-of-the-sle-prospective-observational-cohort-study-spocs-us-data/