Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is widely prescribed in rheumatology, particularly for systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Though generally safe, HCQ can cause rare but serious antimalarial-induced cardiotoxicity (AMIC). AMIC diagnosis is challenging, as cardiac biopsy with electron microscopy (EM) remains the gold standard but is invasive. This study evaluated screening practices for AMIC among HCQ users, focusing on clinical, imaging, and laboratory assessments performed prior to cardiac biopsy.
Methods: We conducted a retrospective review of patients with HCQ exposure who underwent native heart biopsy at our large academic medical center. Biopsy reports were reviewed for AMIC features (e.g., curvilinear, lamellar, myeloid bodies) or other findings. Structured EHR data were extracted for demographics, diagnoses, HCQ prescription dates, cardiac biomarkers (Troponin-I/T, NT-proBNP, CK-MB, hsCRP), and cardiac testing (EKG, echocardiogram, cardiac MRI) prior to biopsy. HCQ exposure duration was calculated from first prescription to biopsy. Abnormal biomarkers were defined by any elevated result prior to biopsy. Group comparisons used t-tests, Fisher’s exact tests, ANOVA, and Tukey’s HSD post-hoc tests.
Results: Among 73 HCQ users who underwent biopsy, 15 had biopsy-confirmed AMIC and 58 did not. AMIC patients more frequently had SLE (80% vs. 38%, p=0.007), while other baseline characteristics (age, sex, race, HCQ duration) did not differ significantly (Table 1). Mean HCQ duration before biopsy was longer in patients with AMIC on biopsy (283 vs. 205 weeks). Few patients had cardiac biomarker testing prior to biopsy, but abnormalities were more common in AMIC, including elevated troponin-T (63% vs. 35%) and CK-MB (56% vs. 32%), though not statistically significant. Cardiac MRI was also underutilized: 53% in AMIC vs. 35% in non-AMIC. Among non-AMIC patients, only 12 (21%) had EM performed as AMIC was not suspected on toluidine blue staining (Table 2). Most biopsies showed non-specific findings such as hypertrophy (74%), fibrosis (45%), and vacuolization (21%).
Conclusion: This study provides one of the largest biopsy-confirmed AMIC case series to date, comparing findings to those of patients taking HCQ who had cardiac biopsies in which AMIC was not confirmed. Few patients underwent cardiac biomarker testing or advanced imaging before biopsy, revealing missed opportunities for earlier detection. These findings underscore the need for non-invasive AMIC screening strategies to improve recognition, estimate disease burden, and enable timely intervention.
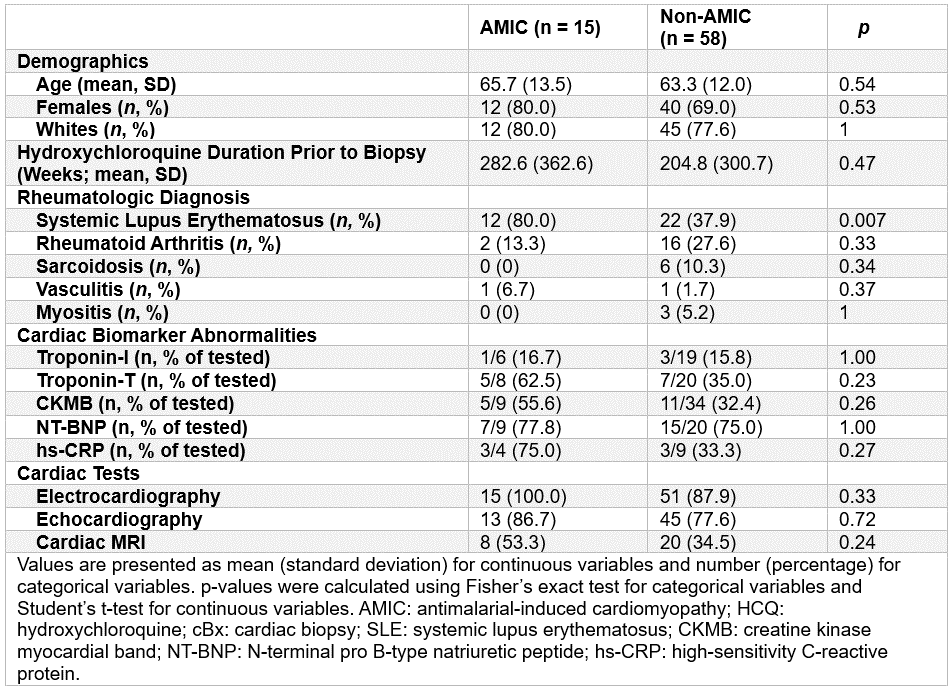 Table 1. Demographic, clinical, laboratory, and cardiac imaging characteristics of 73 patients with biopsy-confirmed antimalarial-induced cardiomyopathy (AMIC) and patients without AMIC (non-AMIC)
Table 1. Demographic, clinical, laboratory, and cardiac imaging characteristics of 73 patients with biopsy-confirmed antimalarial-induced cardiomyopathy (AMIC) and patients without AMIC (non-AMIC)
.gif) Table 2. Features of Cardiac Biopsies in Patients Without Confirmed AMIC (n = 58)
Table 2. Features of Cardiac Biopsies in Patients Without Confirmed AMIC (n = 58)
To cite this abstract in AMA style:
Kim Y, Cabello N, Padera R, Weber B, Costenbader K. Clinical and Laboratory Factors Associated With Biopsy-Confirmed Antimalarial-Induced Cardiomyopathy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/clinical-and-laboratory-factors-associated-with-biopsy-confirmed-antimalarial-induced-cardiomyopathy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-and-laboratory-factors-associated-with-biopsy-confirmed-antimalarial-induced-cardiomyopathy/
