Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Treatment of lupus (or SLE) is complex, especially for young patients who are committed to take hydroxychloroquine (HCQ) lifelong, which carries some risk, albeit low, of eye toxicity. Patients face difficult decisions balancing risks vs. benefits, and decision-making is even harder for patients with limited literacy and English proficiency (LEP). Unable to weigh benefits vs. rare risks of HCQ, patients with limited literacy and LEP may have greater risk aversion leading to nonadherence and worse outcomes contributing to health disparities in lupus. Shared decision-making (SDM) tools could address this problem by supporting patient-clinician communication, and patient’s participation in decision-making. We examined the efficacy of a tool (HCQ-SAFE©) to engage patients in SDM to resolve decisional conflicts around HCQ in SLE.
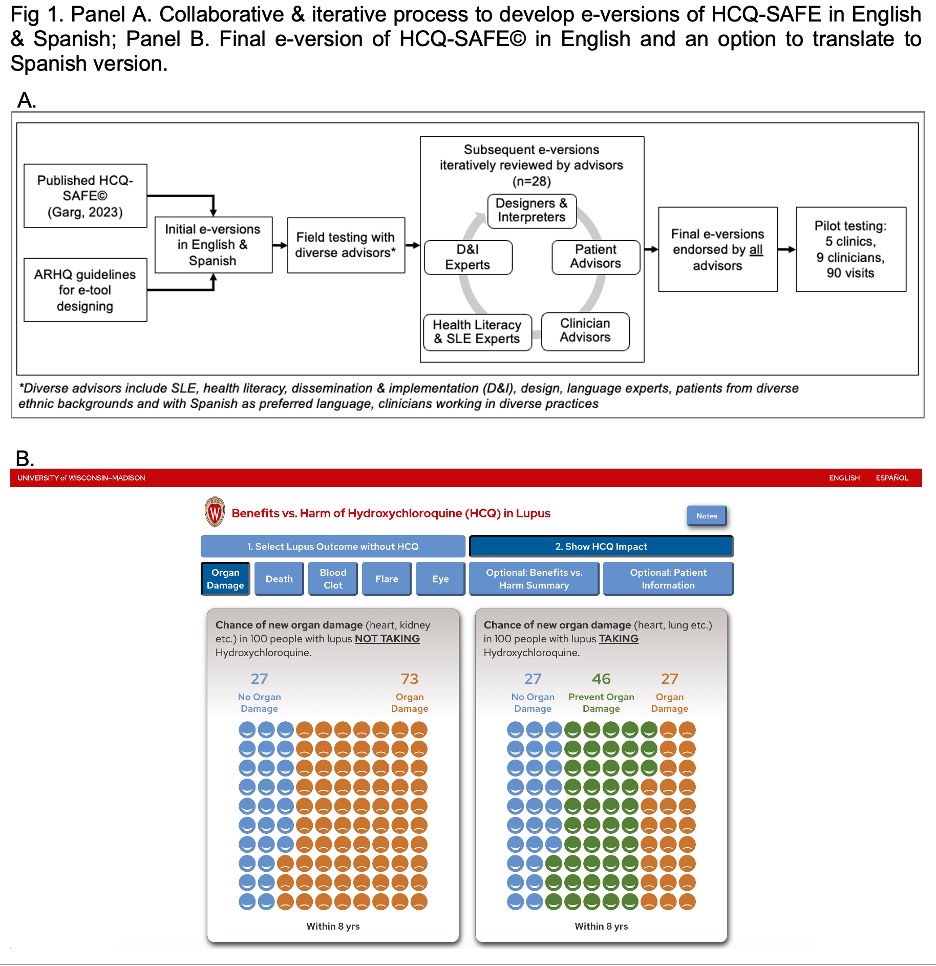
Methods: HCQ-SAFE© is a 5-domain pictorial paper tool that highlights benefits vs. harms of HCQ to support SDM (Garg 2023). In this study, we first developed culturally appropriate e-versions of HCQ-SAFE© in English and Spanish via a collaborative process involving 28 advisors: patients from multiethnic backgrounds and LEP (n=12), clinicians working in diverse practices (n=8), implementation scientists (n=2), designers (n=2), interpreters (n=2), & health literacy experts (n=2). We used an iterative process to revise subsequent prototypes to incorporate advisors’ feedback and deliver final e-versions in English & Spanish which were endorsed by all advisors (Fig 1A-B).
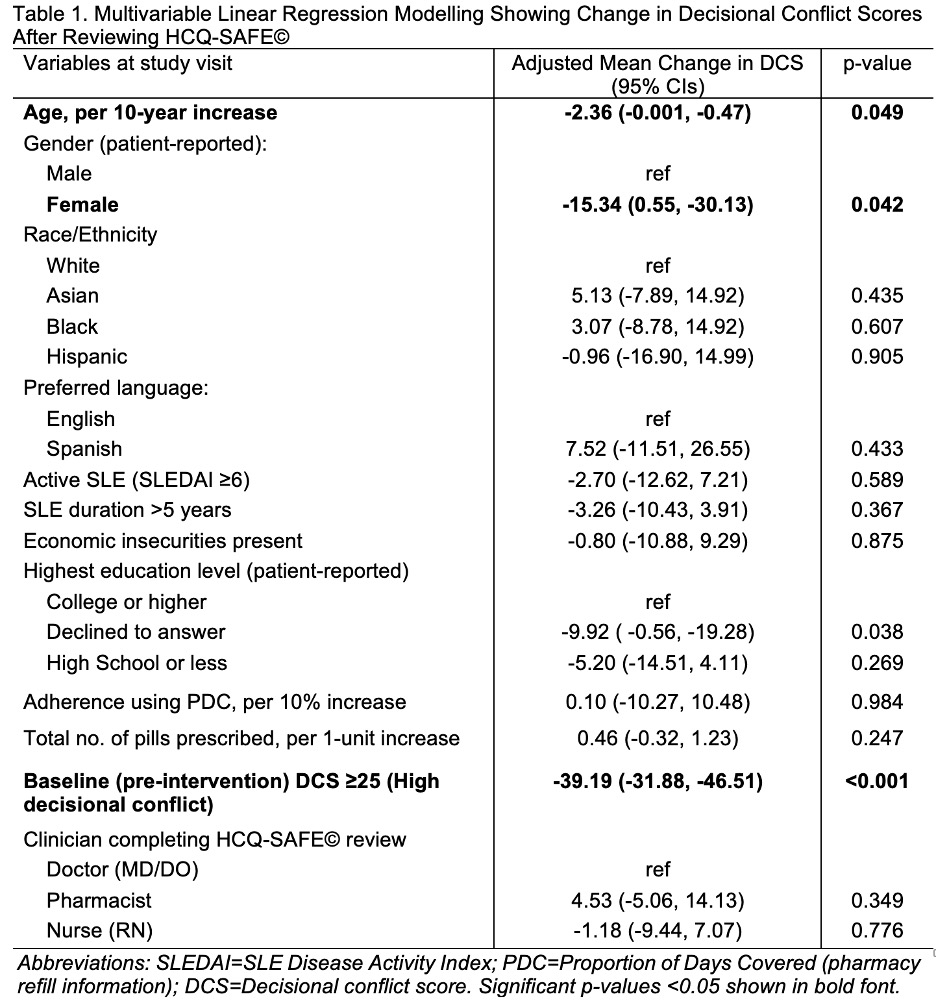
To test the effectiveness of e-versions of HCQ-SAFE© in addressing decisional conflicts, we recruited adults with SLE on HCQ across 5 clinics. We used low literacy version of decisional conflict scale (DCS) to calculate DCS scores (range: 0 (best) – 100 (worst); scores ≥25 indicate clinically significant residual decisional conflict). We compared change in DCS scores pre- vs. post-intervention using linear regression adjusting for covariables (e.g., education, preferred language, clinician type, binary status of high DCS ≥25 at baseline).
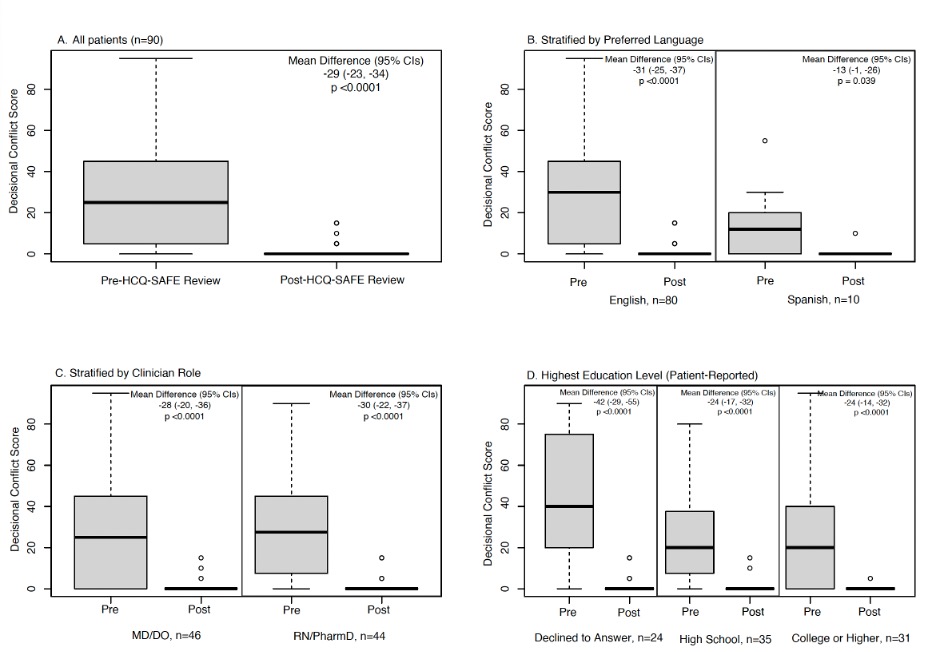
Results: Among 90 participants, 90% were women, 12% preferred Spanish, 54% had high decisional conflicts at baseline. MDs, RNs, and pharmacists completed 51%, 12%, and 37% completed SDM during visits. After reviewing HCQ-SAFE©, a 29-point reduction in DCS scores was seen in all patients (p < 0.001; Fig 2A). Significant reductions were seen in DCS scores when stratified by language (Fig 2B), clinician type (Fig 2C), & highest education level (Fig 2D). In patients with high DCS (≥25) at baseline, a 49-point decrease in DCS (95% CIs -42, -54, p < 0.0001) was noted after reviewing HCQ-SAFE©. Even after adjusting, a 39-point reduction in DCS was noted post-intervention in patients with high DCS at baseline (Table 1). Median time spent by clinicians to review HCQ-SAFE© during visits was 5 mins (IQR 5-10).
Conclusion: Our HCQ-SAFE© tool provides clear, concise, pictorial information, that improved patient understanding and reduced decisional conflicts across diverse populations, including those who are at higher risk of poor outcomes. SDM tools like HCQ-SAFE© can effectively mitigate issues arising from linguistic clarity and lack of decision support.
To cite this abstract in AMA style:
Garg S, Patel J, Ferguson S, Chewning B, Gomez S, keevil J, Gazeley D, Tellez-Giron P, Bartels C. Clarifying Misbeliefs & Resolving Decisional Conflicts About Hydroxychloroquine (HCQ) Through a Shared Decision-Making Tool (HCQ-SAFE©) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clarifying-misbeliefs-resolving-decisional-conflicts-about-hydroxychloroquine-hcq-through-a-shared-decision-making-tool-hcq-safe/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clarifying-misbeliefs-resolving-decisional-conflicts-about-hydroxychloroquine-hcq-through-a-shared-decision-making-tool-hcq-safe/