Session Information
Date: Sunday, October 26, 2025
Title: (0067–0097) Rheumatoid Arthritis – Etiology and Pathogenesis Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Autoantibodies against citrullinated proteins (ACPAs) are diagnostic of rheumatoid arthritis (RA), an autoimmune disease primarily affecting synovial joints. Proteomic analyses have identified human serum albumin as a target of citrullination in RA, and anti-citrullinated albumin antibodies have been reported. This study aimed to characterize the extent and immunological relevance of albumin citrullination in RA.
Methods: Sera from individuals with RA (n = 87), SLE (n = 24), and healthy controls (n = 33) were obtained from the UW Rheumatology Biorepository, and neutrophils were isolated from freshly collected blood samples of RA patients. Anti-CCP status in RA sera was confirmed by ELISA. Neutrophils from RA blood or recombinant PAD2/PAD4 enzymes were used to citrullinate human albumin in vitro. Citrullination was assessed by AMC blotting and LC-MS/MS after in-gel tryptic digestion. Citrullinated and unmodified peptide pairs (14 sites) were tested by ELISA for IgG binding; responses above the 95th percentile of controls were considered positive. Thyroxine binding to native versus citrullinated albumin was measured using competitive ELISA. All protocols were IRB-approved. Mann-Whitney U tests were used for statistical analyses, with p< 0.05 considered significant.
Results: This study analyzed serum from 87 RA patients, confirming CCP positivity in 59.5%, and identified 17 citrullinated arginine residues in albumin from both RA patients and healthy donors using LC-MS/MS. Surprisingly, the stoichiometry of citrullination was similar in both groups, suggesting it is a physiological process. Increased citrullination was observed only in RA synovial fluid, albeit at the same sites. The citrullinated residues were surface-exposed and located near ligand-binding pockets, including R81 and R521 near the FcRn binding interface, indicating potential structural or functional consequences. In vitro, PAD2, PAD4, and live RA neutrophils rapidly citrullinated albumin at multiple sites, with PAD4 inducing the most extensive changes. Citrullination significantly reduced thyroxin binding by 64%, suggesting a regulatory role of citrullination in ligand binding. However, ELISA assays showed minimal IgG ACPA reactivity to either physiologically citrullinated albumin or synthetic citrullinated peptides, with only a few RA sera slightly exceeding control thresholds, likely due to cross-reactivity of ACPA against other citrullinated proteins. These findings suggest that albumin citrullination is a physiological process and not a major immune target in RA.
Conclusion: Albumin citrullination appears to be a physiological process and is not immunogenic in RA, with similar stoichiometry to healthy controls and minimal ACPA recognition. We conclude that citrullinated albumin is unlikely to contribute significantly to ACPA responses in RA and that immunological tolerance against citrullinated albumin is maintained in RA.
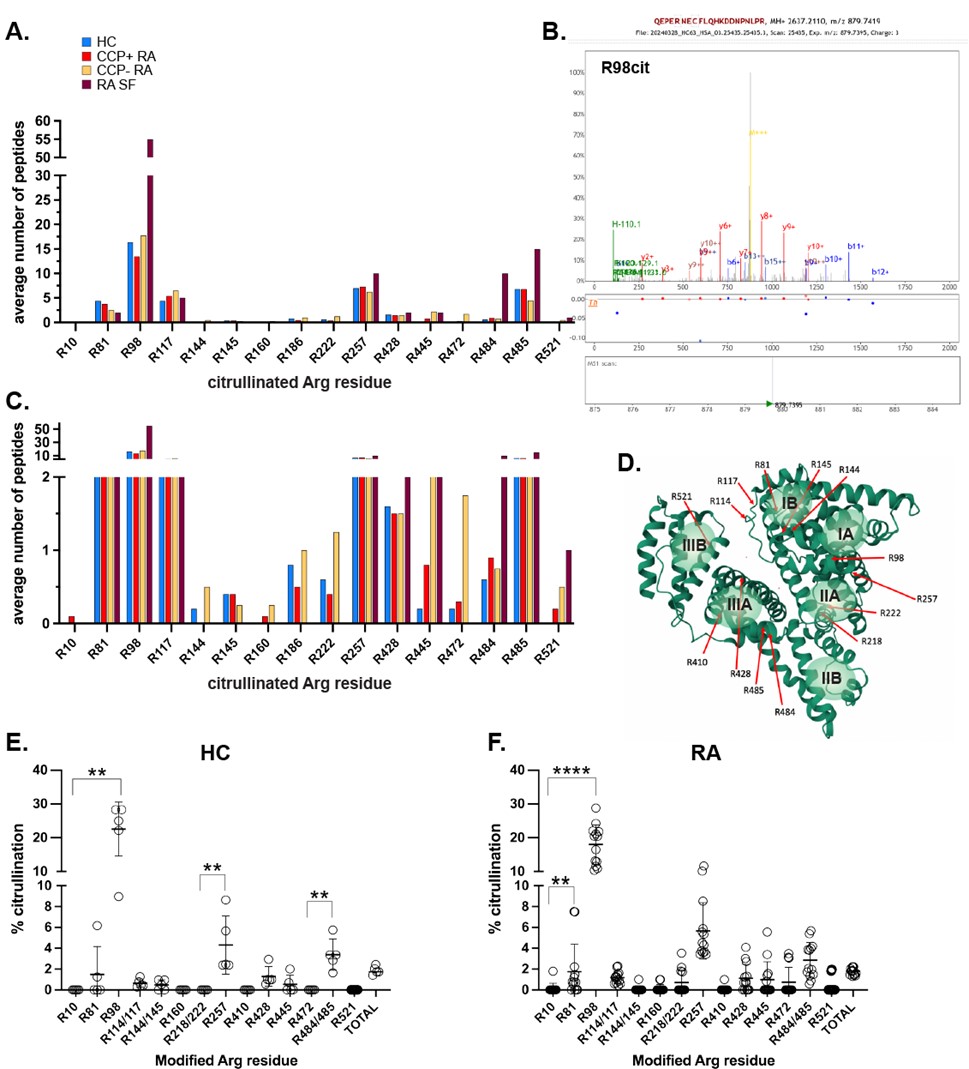 Fig. 1. Summary of mass spectrometry analyses to identify citrullinated Arg residues in albumin. A., Identified sites by the average number of peptides detected in LC-MS/MS experiments in healthy controls (HC, blue bars), CCP-positive RA patients (red bars), CCP-negative RA patients (golden bars), and in synovial fluid from an RA patient (purple bars). B., Mass spectrum for the peptide containing citrullinated R98. C. Same data as in panel A., but with the bottom half of the Y-axis magnified to better visualize the lowest peptide quantities. D., Location of the modified Arg residues in the three-dimensional structure of albumin. The 6 ligand binding pockets are indicated as pale green spheres. E., stoichiometry of Arg citrullination in albumin from healthy donors (HC; n=5) and RA patients (n=13). Statistical significance was assessed using the Mann-Whitney U test. **, p < 0.01, ****, p < 0.001.
Fig. 1. Summary of mass spectrometry analyses to identify citrullinated Arg residues in albumin. A., Identified sites by the average number of peptides detected in LC-MS/MS experiments in healthy controls (HC, blue bars), CCP-positive RA patients (red bars), CCP-negative RA patients (golden bars), and in synovial fluid from an RA patient (purple bars). B., Mass spectrum for the peptide containing citrullinated R98. C. Same data as in panel A., but with the bottom half of the Y-axis magnified to better visualize the lowest peptide quantities. D., Location of the modified Arg residues in the three-dimensional structure of albumin. The 6 ligand binding pockets are indicated as pale green spheres. E., stoichiometry of Arg citrullination in albumin from healthy donors (HC; n=5) and RA patients (n=13). Statistical significance was assessed using the Mann-Whitney U test. **, p < 0.01, ****, p < 0.001.
.jpg) Fig. 2. Citrullination of albumin by PAD2, PAD4, and live neutrophils. A., Stoichiometry of citrullination of albumin at all sites after treatment without (blue) or with PAD2 (golden), PAD4 (green), or both enzymes (tan). B., Arg residues in albumin citrullinated (red) by live neutrophils. C., Numbers of citrullinated sites and peptides in panel B. D., Binding of thyroxin (T4) by albumin treated with (green) or without (brown) PAD4, as indicated. Statistical significance was assessed using the Mann-Whitney U test. E., Anti-modified citrulline (AMC) of albumin treated with PAD4, with citrullinated fibrinogen as a positive control.
Fig. 2. Citrullination of albumin by PAD2, PAD4, and live neutrophils. A., Stoichiometry of citrullination of albumin at all sites after treatment without (blue) or with PAD2 (golden), PAD4 (green), or both enzymes (tan). B., Arg residues in albumin citrullinated (red) by live neutrophils. C., Numbers of citrullinated sites and peptides in panel B. D., Binding of thyroxin (T4) by albumin treated with (green) or without (brown) PAD4, as indicated. Statistical significance was assessed using the Mann-Whitney U test. E., Anti-modified citrulline (AMC) of albumin treated with PAD4, with citrullinated fibrinogen as a positive control.
.jpg) Fig. 3. ELISAs with intact albumin and citrullinated albumin peptides. A., Detection by ELISA of IgG antibodies binding purified albumin. B., Positive control ELISA with the same serum samples detecting IgG binding to citrullinated fibrinogen. Statistical significance was assessed using the Mann-Whitney test. C., Sequence of all peptides used in ELISAs. The unmodified Arg residues are indicated in blue and the citrulline residues in red. D., IgG reactivity in RA sera (n=87) against the indicated citrullinated peptides after subtraction of the reactivity against the unmodified peptide. E., The exact same ELISA using healthy control (HC) sera (n=33). The 95th percentile of the HC distribution for each peptide is indicated as a horizontal dotted line. Statistical significance was assessed using the Mann-Whitney U test between reactivity for each peptide between HC and RA. Note that the statistically significant values all represent lower reactivity in RA than in HC. *, p < 0.5; **, p < 0.01; ***, p < 0.005, ****, p < 0.001.
Fig. 3. ELISAs with intact albumin and citrullinated albumin peptides. A., Detection by ELISA of IgG antibodies binding purified albumin. B., Positive control ELISA with the same serum samples detecting IgG binding to citrullinated fibrinogen. Statistical significance was assessed using the Mann-Whitney test. C., Sequence of all peptides used in ELISAs. The unmodified Arg residues are indicated in blue and the citrulline residues in red. D., IgG reactivity in RA sera (n=87) against the indicated citrullinated peptides after subtraction of the reactivity against the unmodified peptide. E., The exact same ELISA using healthy control (HC) sera (n=33). The 95th percentile of the HC distribution for each peptide is indicated as a horizontal dotted line. Statistical significance was assessed using the Mann-Whitney U test between reactivity for each peptide between HC and RA. Note that the statistically significant values all represent lower reactivity in RA than in HC. *, p < 0.5; **, p < 0.01; ***, p < 0.005, ****, p < 0.001.
To cite this abstract in AMA style:
Shaikh F, mustelin c, Wang X, Mustelin T. Citrullinated Serum Albumin Is Not an Autoantigen in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/citrullinated-serum-albumin-is-not-an-autoantigen-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/citrullinated-serum-albumin-is-not-an-autoantigen-in-rheumatoid-arthritis/
