Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Cigarette smoking has been epidemiologically linked to the development of ACPA+ rheumatoid arthritis (RA) and, by extension, RA-associated interstitial lung disease (RA-ILD). Providing strong evidence for the contribution of gene-environment interactions to disease pathogenesis, the risk for ACPA+ RA is increased more than 20 fold in cigarette smokers who also possess HLA-DRB1*0401 or other shared epitope alleles. This link between smoking, the shared epitope, and risk of RA/RA-ILD is thought to arise from immune responses targeting in situ, smoking-induced post-translational modifications (PTMs) such as citrullination. However, because most of the data linking smoking and PTMs has been “associative,” we examined the ability of cigarette smoke to directly induce PTMs in both in vitro and in vivo model systems.
Methods: Primary human bronchial epithelial cells (HBEs) were isolated, cultured at the air-liquid interface, and robotically exposed to defined doses of cigarette smoke vs. air prior to harvesting and preparation of cell extracts. Following SDS-PAGE, separated proteins were transferred to nitrocellulose membranes and probed with anti-modified citrulline antibody (EMD Millipore). Alternatively, extracts were transferred directly to membranes and probed with polyclonal antibody recognizing malondialdehyde-acetaldehyde (MAA)-modified proteins. Complementing these in vitro studies, HLA-DR4 transgenic (DR4) and C57BL/6 (WT) mice were exposed to daily cigarette smoke for 2 months and then harvested for immunofluorescence (IF) staining of lung tissue and ELISA-based analysis of PTM-targeted autoantibody production. Lung tissue from WT mice with bleomycin-induced pulmonary fibrosis was used as a control for PTM formation.
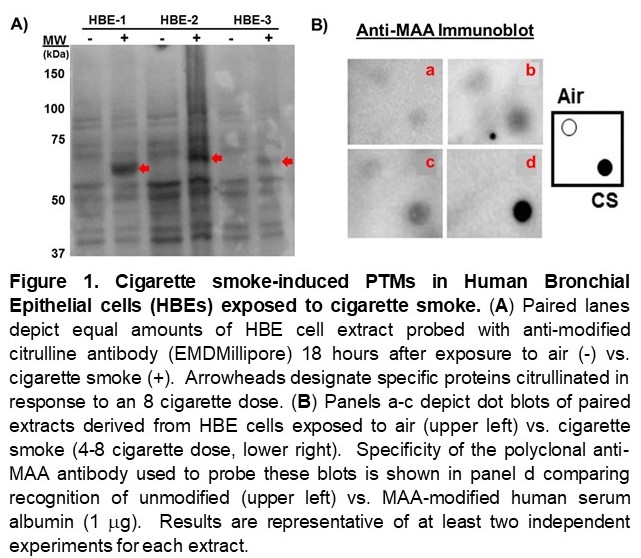
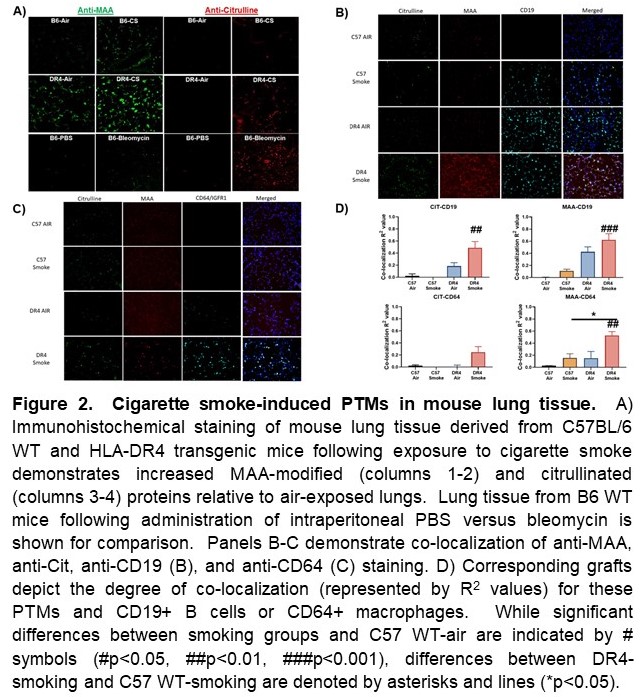
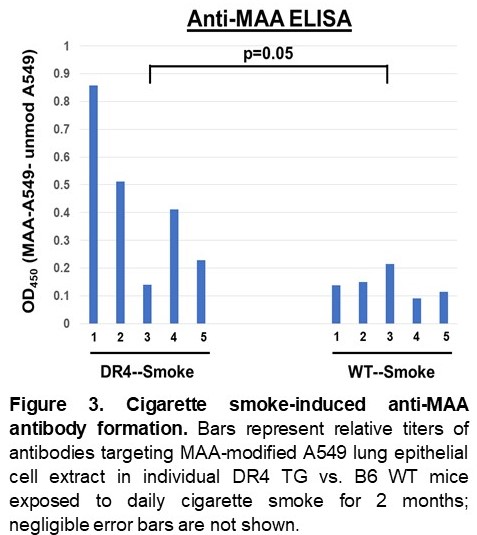
Results: Immunoblots of HBE cell extracts demonstrated smoke-induced citrullination as well as MAA-modification (Figure 1). Similarly, in vivo administration of cigarette smoke induced both citrullination and MAA modification of lung tissue, particularly in DR4 TG mice (Figure 2A). Additional IF staining revealed that these PTMs co-localized with CD19+ B cells and CD64+ macrophages (Figure 2B-D), both of which were increased in the lung tissue of cigarette smoke-exposed DR4 TG mice (p< 0.01 for CD19+ cells and < 0.0001 for CD64+ cells, DR4 TG-smoke vs. WT-smoke). ELISAs also demonstrated preferential formation of anti-MAA antibodies in the serum of DR4 TG mice exposed to cigarette smoke (Figure 3), but a limited profile of ACPA were not detected. Mice showed no evidence of overt arthritis in any of the treatment groups.
Conclusion: For the first time, these studies directly demonstrate that cigarette smoke can induce PTMs such as citrullination and MAA modification in lung tissue/cells. Corresponding development of PTM-targeted immune responses in DR4 TG mice support a paradigm in which cigarette smoke triggers a breach of immune tolerance in immunogenetically predisposed hosts as a foundation for the development of RA/RA-ILD.
To cite this abstract in AMA style:
Gregory A, Kliment C, Thiele G, Duryee M, Mikuls T, England B, Poole J, Ascherman D. Cigarette Smoking Induces Post-translational Protein Modifications in Both in Vitro and in Vivo Models of Rheumatoid Arthritis-associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/cigarette-smoking-induces-post-translational-protein-modifications-in-both-in-vitro-and-in-vivo-models-of-rheumatoid-arthritis-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cigarette-smoking-induces-post-translational-protein-modifications-in-both-in-vitro-and-in-vivo-models-of-rheumatoid-arthritis-associated-interstitial-lung-disease/