Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Janus kinase inhibitors (JAKi) are a widely acceptable drug class in the treatment of RA. However, real-world data on the patterns of JAKi use and effectiveness are limited, particularly those describing therapeutic choice after initial JAKi use, including JAKi cycling. This study sought to characterize JAKi initiators who switch to a subsequent therapy and to examine factors associated with the use of another JAKi, or a therapy with a different mechanism of action (MoA).
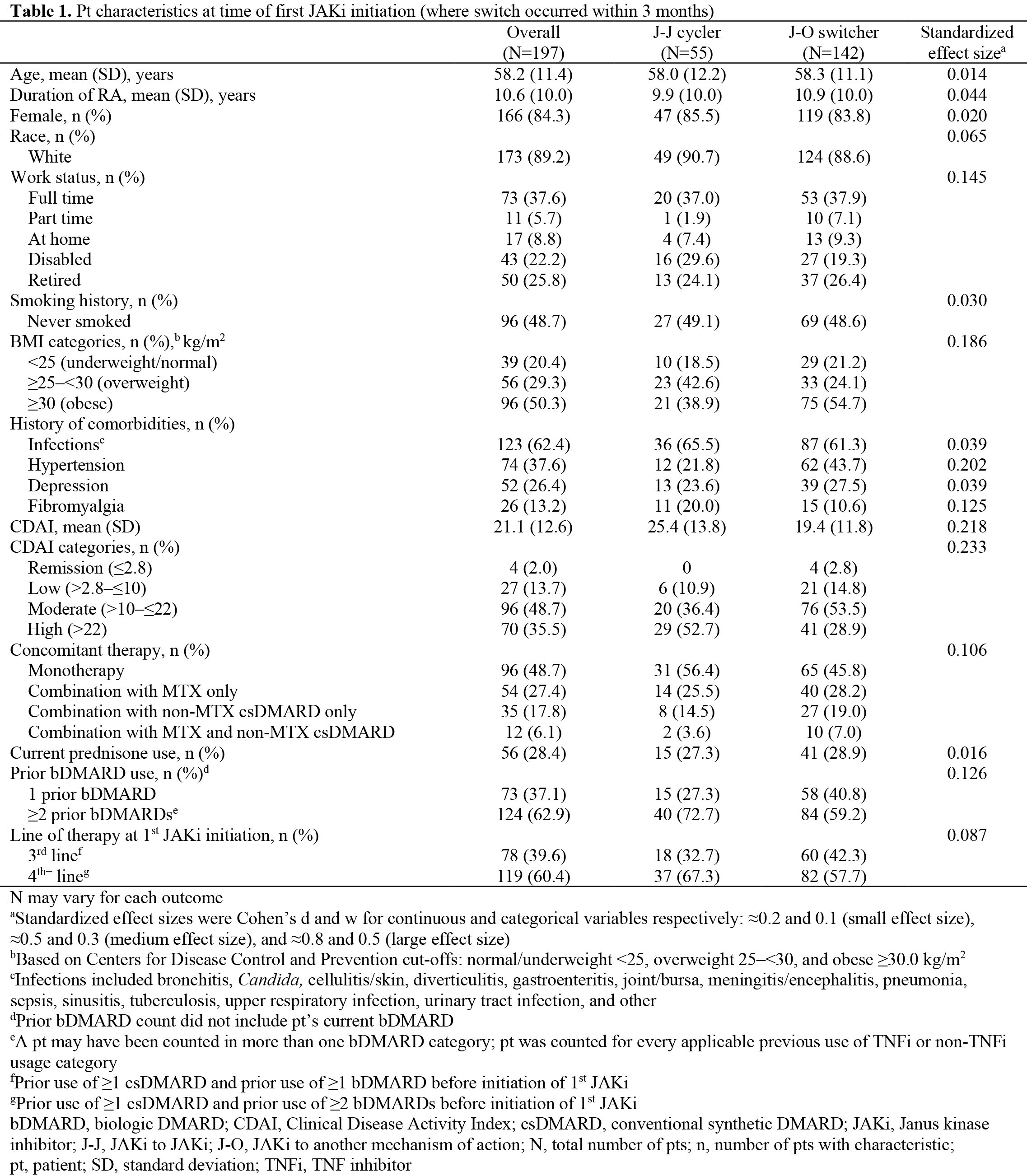
Methods: This study included patients (pts) with RA enrolled in the United States CorEvitas RA Registry who initiated a JAKi from 8/16/2019–10/30/2023, who had prior exposure to ≥1 conventional synthetic and ≥1 biologic (b)DMARD, who switched to another therapy within 3 months of JAKi discontinuation and had a Clinical Disease Activity Index (CDAI) record at JAKi initiation and switch. Pts were classified as JAKi cyclers (‘J-J cyclers’) or JAKi switchers to another MoA (‘J-O switchers’). Clinical outcomes (including CDAI and achievement of CDAI low disease activity [LDA]) and pt-reported outcomes (PROs, including Pt Global Assessment, fatigue, pain, and HAQ-Disability Index) were evaluated at initial JAKi initiation and time of therapy cycle/switch. Factors associated with switching to another MoA were evaluated using multivariable logistic regression, adjusted for a priori-selected covariates (age, sex, and CDAI at time of JAKi initiation) and additional covariates demonstrated to be different between groups at baseline (effect size >0.10 and ≤5% missingness).
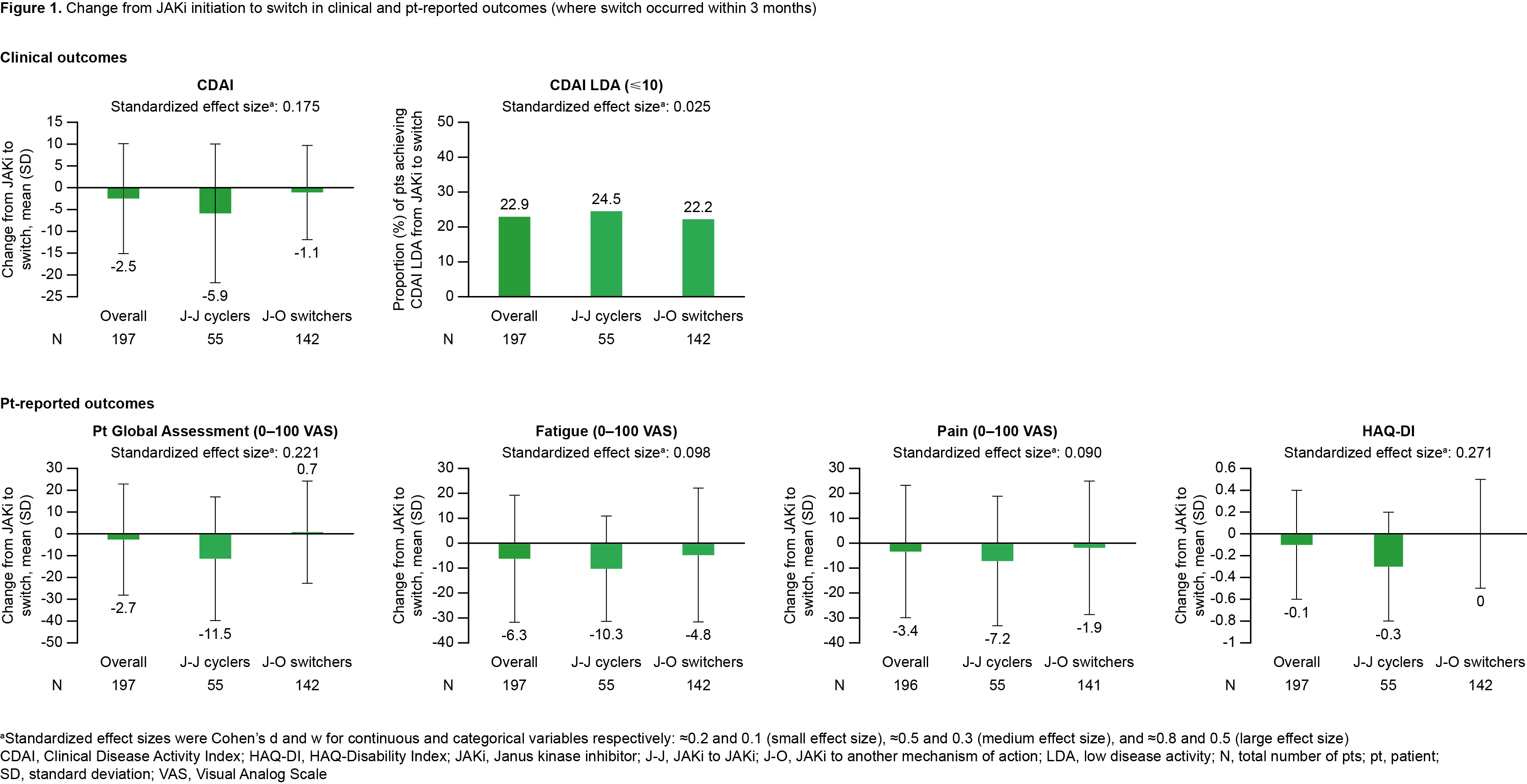
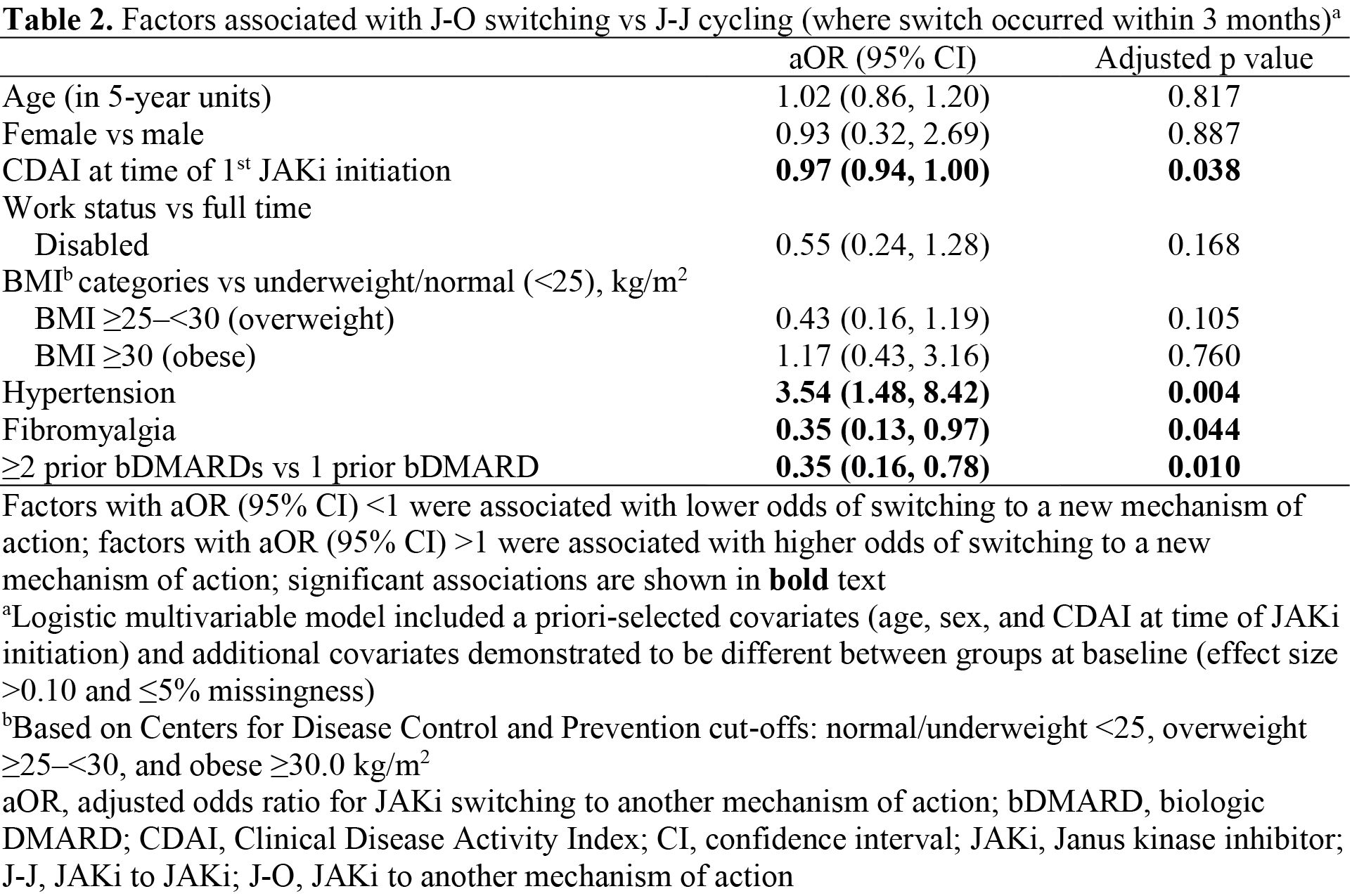
Results: Overall, 197 pts were included. Of these, 27.9% (N=55) pts were J-J cyclers and 72.1% (N=142) were J-O switchers. Most pts had moderate/high CDAI (84.3%) and received the initial JAKi as 4th+ line of therapy (60.4%; Table 1). For J-O switchers, TNF inhibitors were the most common next therapy, regardless of initial JAKi. From JAKi initiation to time of switch, mean improvements in disease activity and PROs were consistently numerically greater for J-J cyclers vs J-O switchers. Mean change (standard deviation [SD]) in CDAI between JAKi initiation and switch was ‑5.9 (15.9) for J-J cyclers and ‑1.1 (10.8) for J-O switchers, while 24.5% of J-J cyclers and 22.2% of J-O switchers achieved CDAI LDA between JAKi initiation and switch. Mean change (SD) in Pt Global Assessment was ‑11.5 (28.4) for J-J cyclers and 0.7 (23.5) for J-O switchers (Figure 1). A history of hypertension was associated with higher odds of switching to a new MoA, while a history of fibromyalgia and prior use of ≥2 bDMARDs were associated with lower odds of switching to a new MoA (Table 2).
Conclusion: These results suggest that J-J cycling is common among JAKi users who switch treatment. J-J cycling is more likely in those with a history of fibromyalgia and ≥2 vs 1 prior bDMARD use and occurs among pts who experience better treatment response to the initial JAKi. While this analysis provides a detailed overview of JAKi use in a North American RA pt population, more research regarding JAKi cycling and factors associated with cycling in other pt populations is warranted.
Acknowledgments: Study sponsored by Pfizer and registry by CorEvitas, LLC. Medical writing support provided by Lewis C Rodgers, CMC Connect; funded by Pfizer.
To cite this abstract in AMA style:
Baker J, Mizelle K, Saikali W, Moore P, Kang H, Gruben D, Shelbaya A, Stockert L, O'Brien J. Characterization of Second-Line Therapy After First Janus Kinase Inhibitor Use: Results from the CorEvitas RA Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/characterization-of-second-line-therapy-after-first-janus-kinase-inhibitor-use-results-from-the-corevitas-ra-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterization-of-second-line-therapy-after-first-janus-kinase-inhibitor-use-results-from-the-corevitas-ra-registry/