Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: In recent decades, the therapeutic arsenal in RA has dramatically expanded. Baricitinib (BARI) and Tofacitinib (TOFA) were the first JAK inhibitors (JAKi) to be recommended for moderate-to-severe RA after conventional synthetic DMARD failure. Several alerts have arisen concerning an increased risk of veinous thromboembolism (VTE) with these drugs. In May 2019, the European Medicines Agency (EMA) sent a warning about an interim post-marketing analysis revealing a significant increase in the number of VTE with TOFA at the dose of 10 mg twice daily. The first objective of this study was to compare the characteristics of patients initiating a JAKi before versus after EMA’s warning to determine if use restrictions were currently applied in daily practice. The second objective was to compare the persistence of BARI and TOFA in real-world.
Methods: A retrospective multicentric cohort study was conducted. Patients with RA were included if they fulfilled the 2010 ACR/EULAR RA classification criteria and initiated BARI or TOFA between October 2017 and September 2020. Patients were JAKi-naïve. Patient characteristics regarding VTE risk factors were compared between the two periods (before and after May 2019) by using pre-specified statistical tests. Comparison of persistence was assessed by using pre-specified propensity-score methods.
Results: 232 patients were included: 155 with BARI and 77 with TOFA (Table 1). Mean age was 59.6±14.2 years old for BARI and 56.7±13.4 years old for TOFA. Median duration of RA was 11 years (IQR, 4 to 20). The proportion of bDMARD-naïve patients was 14.8% with BARI and 9.1% with TOFA. Combination with a csDMARD was reported in 38.7 % (BARI) and 37.4 % (TOFA) of patients. The baseline characteristics of patients regarding VTE risk factors were not statistically different when JAKi was initiated before versus after EMA’s warnings although a trend towards a lower proportion of thromboembolic personal history was observed (Table 2). The overall median persistence was 17 months (IQR, 13 to 22). Persistence rate at 2 years was 39.3% (BARI) and 42.8% (TOFA) with no significant difference between the two drugs in the propensity score analysis: hazard ratio 0.96; 95% Confidence Interval: 0.52 to 1.74; p=0.89 (Figure 1). BARI and TOFA were discontinued due to inefficacy in 64% and 60% of cases, respectively. Both drugs were discontinued due to an adverse event in 30.7% (BARI) and 31.4% (TOFA) of cases. 5 VTE events occurred during our study, 4 with BARI, 1 with TOFA. 4/5 events occurred in patients who initiated JAKi before May 2019. There has been no incident neoplasia nor myocardial infarction reported.
Conclusion: In conclusion, our study revealed that EMA’s warnings have not significantly changed RA patient characteristics initiating a JAKi. BARI and TOFA have a similar persistence in our study. The tolerance profile is consistent with post-marketing and real-world data. VTE risk remains a concern in current practice. This study provides additional data and is a hot topic pending the publication of the ORAL-surveillance study (NCT02092467). Monitoring of new marketed JAKi will help understand if there are specific efficacy and safety profiles among JAKi according to the selectively targeted Janus Kinases.
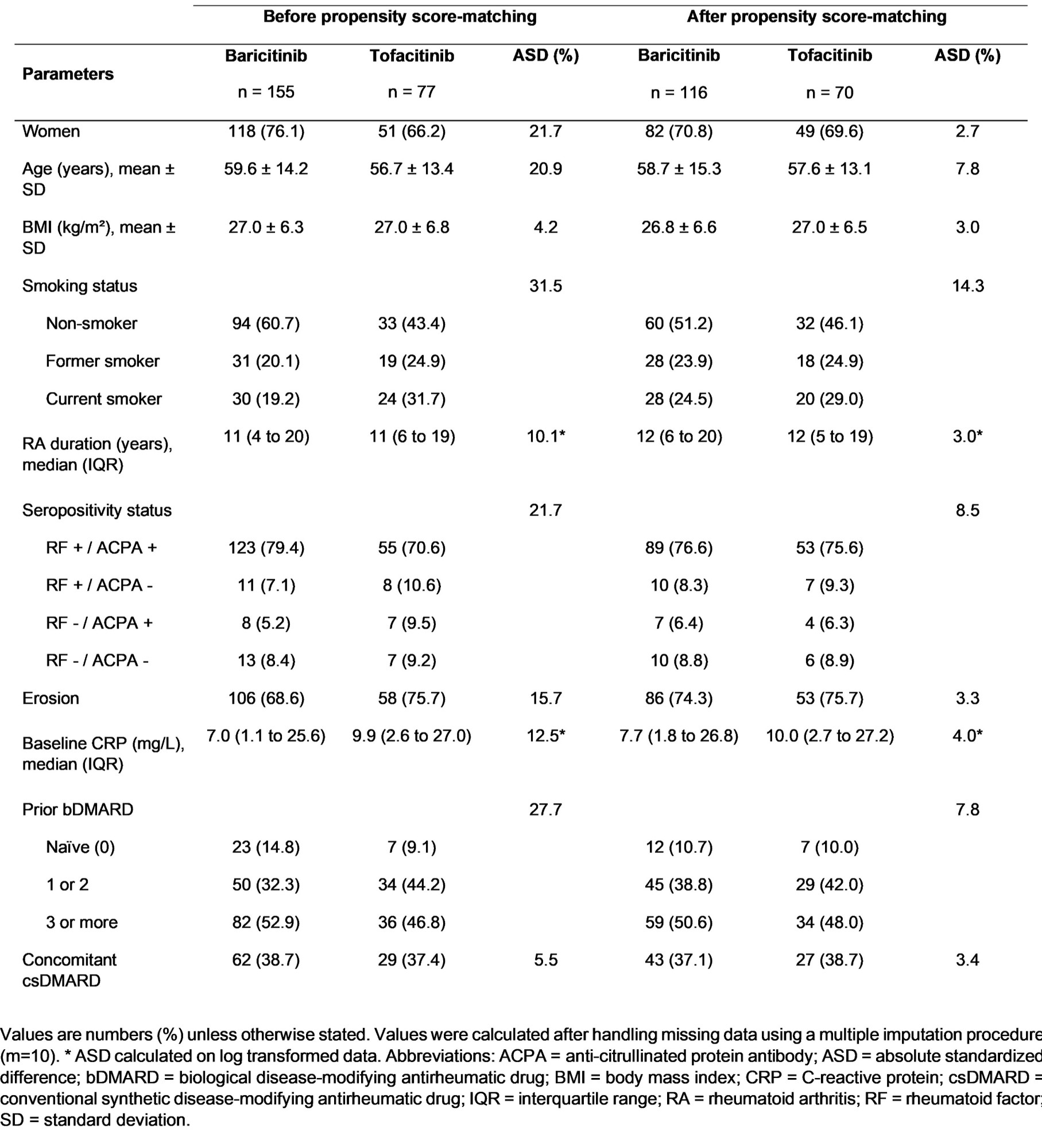 Table 1- Baseline characteristics according to molecules before and after propensity score-matching
Table 1- Baseline characteristics according to molecules before and after propensity score-matching
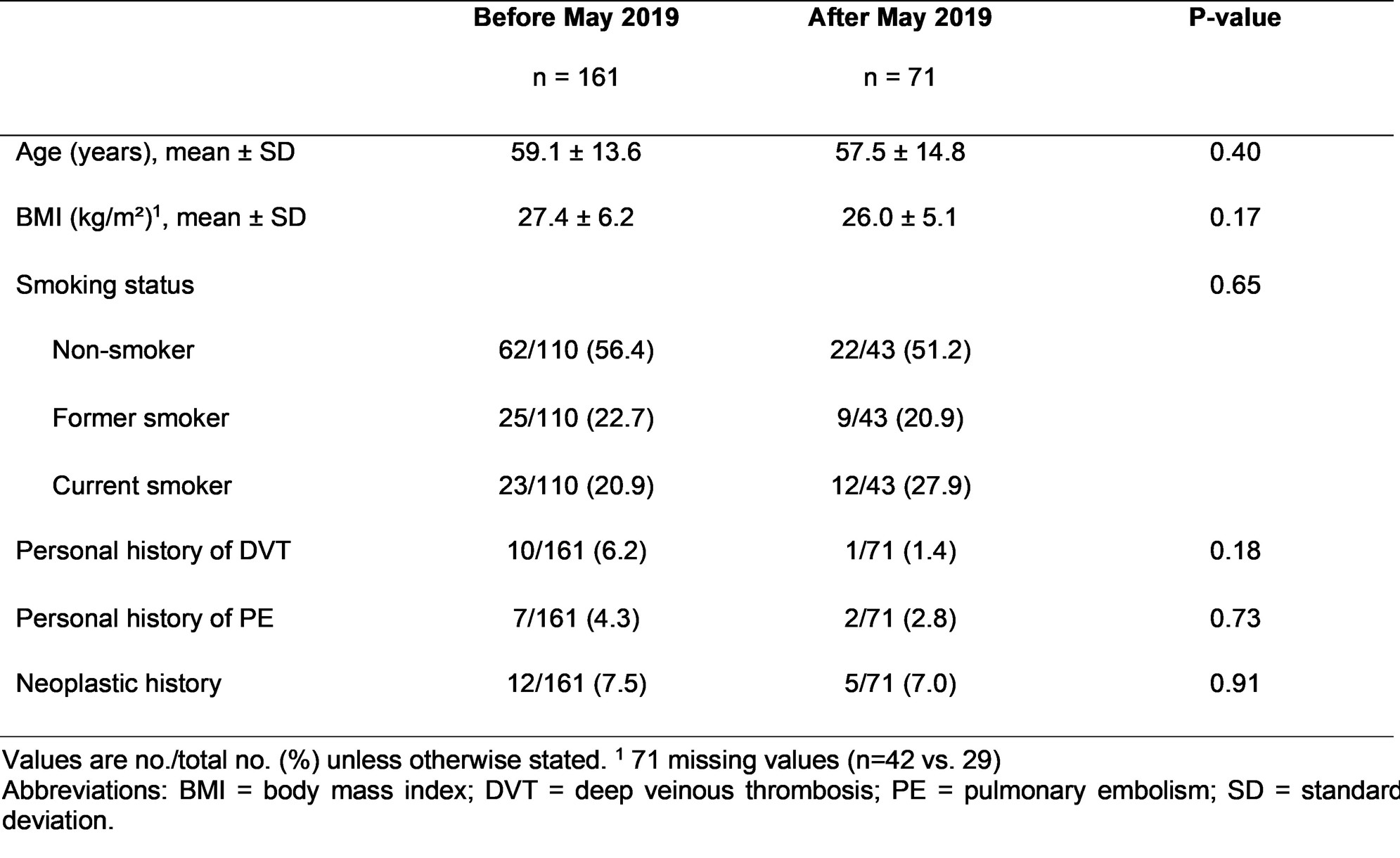 Table 2 – Comparison of the baseline characteristics of patients according to JAKi initiation before versus after May 2019
Table 2 – Comparison of the baseline characteristics of patients according to JAKi initiation before versus after May 2019
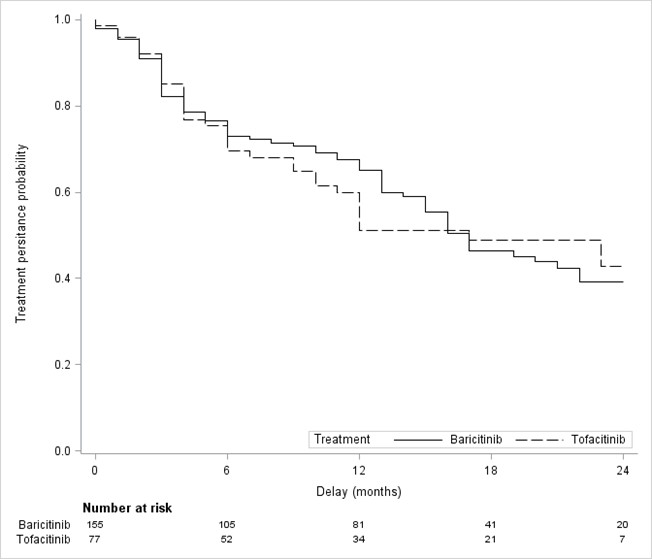 Figure 1 – Two-year follow-up treatment persistence with Baricitinib (BARI) and Tofacitinib (TOFA)
Figure 1 – Two-year follow-up treatment persistence with Baricitinib (BARI) and Tofacitinib (TOFA)
To cite this abstract in AMA style:
PHILIPPOTEAUX C, DEPREZ V, LETAROUILLY J, CAILLIAU E, HOUVENAGEL E, DEPREZ X, NOTTEZ A, PHILIPPE P, PASCART T, GOEB V, FLIPO R. Characteristics of RA Patients Treated with JAK Inhibitors Before versus After VTE Warnings: Results of a Real-World Multicentric Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/characteristics-of-ra-patients-treated-with-jak-inhibitors-before-versus-after-vte-warnings-results-of-a-real-world-multicentric-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-of-ra-patients-treated-with-jak-inhibitors-before-versus-after-vte-warnings-results-of-a-real-world-multicentric-study/
