Session Information
Date: Tuesday, October 28, 2025
Title: (1990–2014) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Gout flares are an important treatment outcome in gout. Although flares are typically assessed by occurrence (yes/no) or a simple count in trials of urate-lowering therapy (ULT), it is possible that certain flare characteristics could also serve as a measure of ULT efficacy. We evaluated gout flare characteristics among trial participants who were started on treat-to-target ULT and assessed the extent to which achievement of serum urate (SU) goal impacted flare characteristics.
Methods: We performed a post-hoc analysis of the STOP Gout trial, a non-inferiority, randomized, double-blind, placebo-controlled trial of allopurinol vs. febuxostat (PMID 35294114). In Phase 1 (0-24 weeks), ULT dose was adjusted to achieve SU goal (i.e., < 6mg/dL or < 5mg/dL with tophi) and prophylaxis was provided (NSAIDs, colchicine, glucocorticoids). In Phase 2 (25-48 weeks), a single ULT dose adjustment was allowed to achieve SU goal. Prophylaxis was discontinued prior to Phase 3 (49-72 weeks). Flares were identified throughout the trial using a modification of validated criteria. Flare characteristics were assessed as pain intensity, number of joints involved, and duration. For those experiencing a flare during all 3 study Phases, characteristics of the first flare in each Phase were compared via generalized linear mixed models with random effects for participants. We next compared characteristics of the first flare in Phase 3 (when participants were off prophylaxis) based upon SU goal achievement at the end of Phase 2. We repeated these analyses limited to those without baseline tophi because the flare criteria may be less sensitive in those with tophi.
Results: Of the 940 STOP Gout participants, 602 (64%) (99% male, mean baseline SU 8.6mg/dL, 19% with tophi) had ≥1 flare during the 72-week study. Flare characteristics were similar across the 3 Phases among the 113 participants with ≥1 flare in each of Phase 1-3 (Figure 1); these results did not change after excluding those with tophi (not shown). Of 835 participants with SU measured at the end of Phase 2, 239/648 (37%) who achieved SU goal and 61/187 (33%) who didn’t achieve SU goal had ≥1 flare in Phase 3. Flares in those who achieved SU goal had significantly lower mean pain intensity, but similar durations and number of joints involved than in those who did not (Figure 2). Among those without baseline tophi, both flare pain intensity and symptom duration were significantly lower with SU goal achievement (vs. not) (Figure 2).
Conclusion: In those who experienced ≥1 flare in all three study phases, the flare characteristics did not change meaningfully over time. However, achievement of SU goal was associated with significantly lower pain intensity and shorter symptom duration among those without tophi. With prior reports suggesting that ≥2 years of oral ULT may be required to meaningfully reduce flares counts, these results suggest that the inclusion of flare characteristics such as pain intensity and symptom duration into trial outcomes could potentially provide insights into efficacy of ULT in a shorter amount of time than conventional studies utilizing flare counts as the outcome.
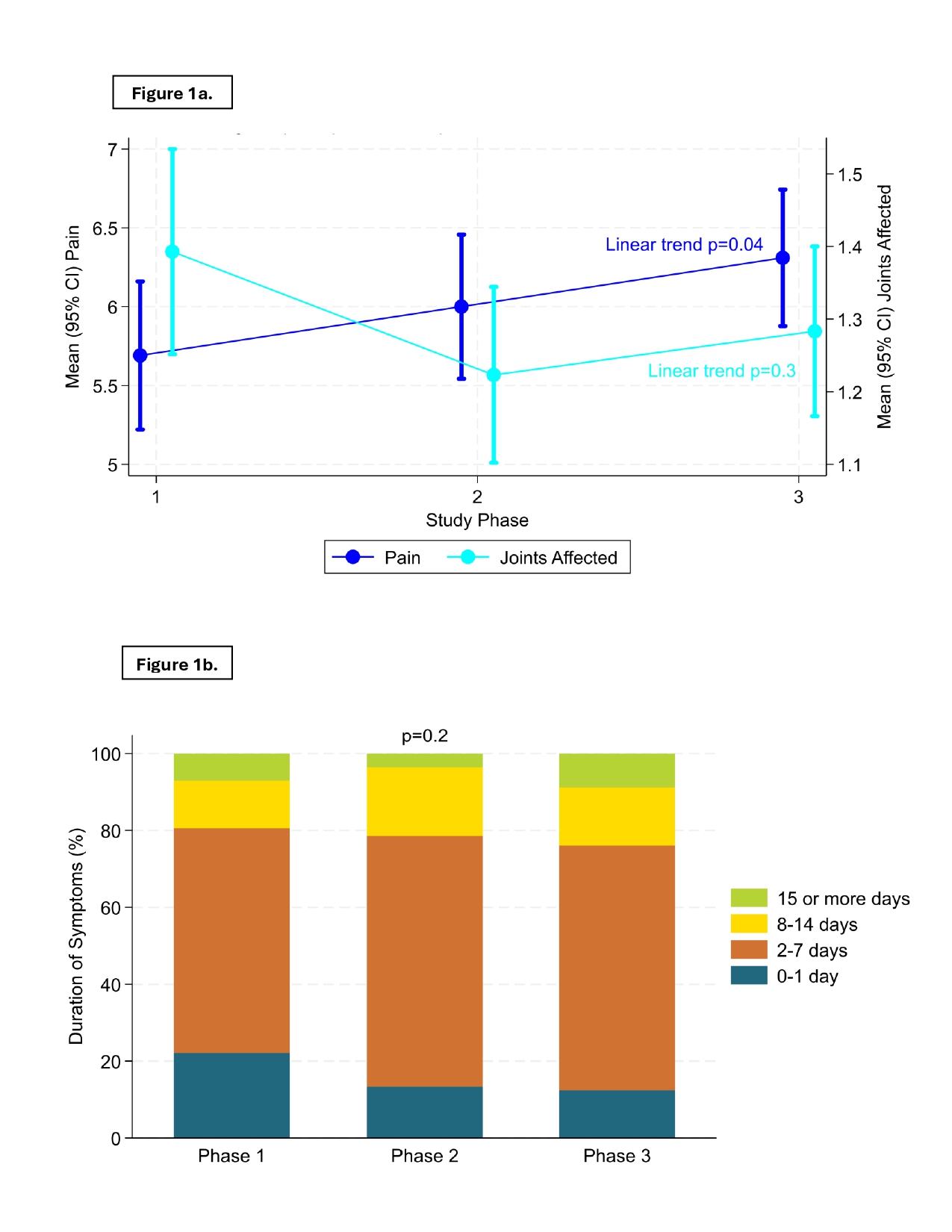 Figure 1a. Mean Pain Intensity and Mean # of Joints Involved for the 1st Flare Within Each Phase in Those who Experienced at least 1 Flare in All Three Phases (n=113). Figure 1b. Duration of Flare Symptoms for the 1st Flare Within Each Phase in Those who Experienced at least 1 Flare in All Three Phases (n=113).
Figure 1a. Mean Pain Intensity and Mean # of Joints Involved for the 1st Flare Within Each Phase in Those who Experienced at least 1 Flare in All Three Phases (n=113). Figure 1b. Duration of Flare Symptoms for the 1st Flare Within Each Phase in Those who Experienced at least 1 Flare in All Three Phases (n=113).
.jpg) Figure 2a. Comparison of Mean Pain Intensity and Mean # of Joints Affected for the 1st Flare in Phase 3 Based on SU Goal Achievement in the Whole Sample (n=300) and in Those Without Baseline Tophi (n=233). Figure 2b. Comparison of Duration of Flare Symptoms for the 1st Flare in Phase 3 Based on SU Goal Achievement in the Whole Sample (n=300) and in Those Without Baseline Tophi (n=233).
Figure 2a. Comparison of Mean Pain Intensity and Mean # of Joints Affected for the 1st Flare in Phase 3 Based on SU Goal Achievement in the Whole Sample (n=300) and in Those Without Baseline Tophi (n=233). Figure 2b. Comparison of Duration of Flare Symptoms for the 1st Flare in Phase 3 Based on SU Goal Achievement in the Whole Sample (n=300) and in Those Without Baseline Tophi (n=233).
To cite this abstract in AMA style:
Yang J, Mikuls T, Sayles H, Pillinger M, Newcomb J, Kramer B, Davis-Karim A, Brophy M, Ferguson R, Palevsky P, O'Dell J, Neogi T. Characteristics of Gout Flares Over Time with Treat-to-Target Urate-Lowering Therapy Use [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/characteristics-of-gout-flares-over-time-with-treat-to-target-urate-lowering-therapy-use/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-of-gout-flares-over-time-with-treat-to-target-urate-lowering-therapy-use/
