Session Information
Date: Saturday, November 16, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Polymyalgia rheumatica (PMR), a common inflammatory rheumatic condition in people aged ≥50 years, is primarily treated with glucocorticoids (GC). Extended GC therapy can increase the risk of GC-related toxicity in this elderly population. Early recognition of patients requiring GC treatment for >1 year will help identify patients who could benefit from GC sparing therapies. The study objective was to identify characteristics at 6 months in new onset PMR patients associated with GC use >1 year.
Methods: This was an exploratory analysis of an inception cohort of PMR patients identified from the US fee-for-service Medicare claims from 10/01/2016 to 12/31/2020 (Curtis J, et al. Arthritis Rheumatol 2023, 75). Patients included had no history of PMR or giant cell arteritis, were ≥50 years, had ≥1 inpatient/≥2 outpatient claims for PMR (ICD-10-CM M35.3) ≥30 days and < 365 days apart. They initiated GC (prednisone equivalent) 7.5–25 mg/day ≤30 days after 1st inpatient code or from 1st outpatient to ≤30 days after 2nd outpatient code, had GC dose ≥200 mg in first ≤30 days and ≥4 months continuous GC use. Continuous enrollment ≥1 year prior to first diagnosis date was required. Patients with other systemic rheumatic disease, malignancy treatment, multiple sclerosis, organ transplant, or prescription for conventional synthetic immunomodulatory drugs [csIM (methotrexate [MTX], leflunomide, azathioprine)] or interleukin-6 receptor inhibitors, ≤1 year prior to first (inpatient) or second (outpatient) PMR diagnosis to 6 months after GC initiation (baseline period), were also excluded (GC cohort). A supplemental analysis evaluated patients who met other criteria and received a csIM ≤6 months from GC initiation (csIM cohort). The primary outcome was comparison of characteristics (demographic, clinical, and healthcare resource utilization) at 6 months between patients who were on GC vs. off GC ( >60-day gap) at 1 year.
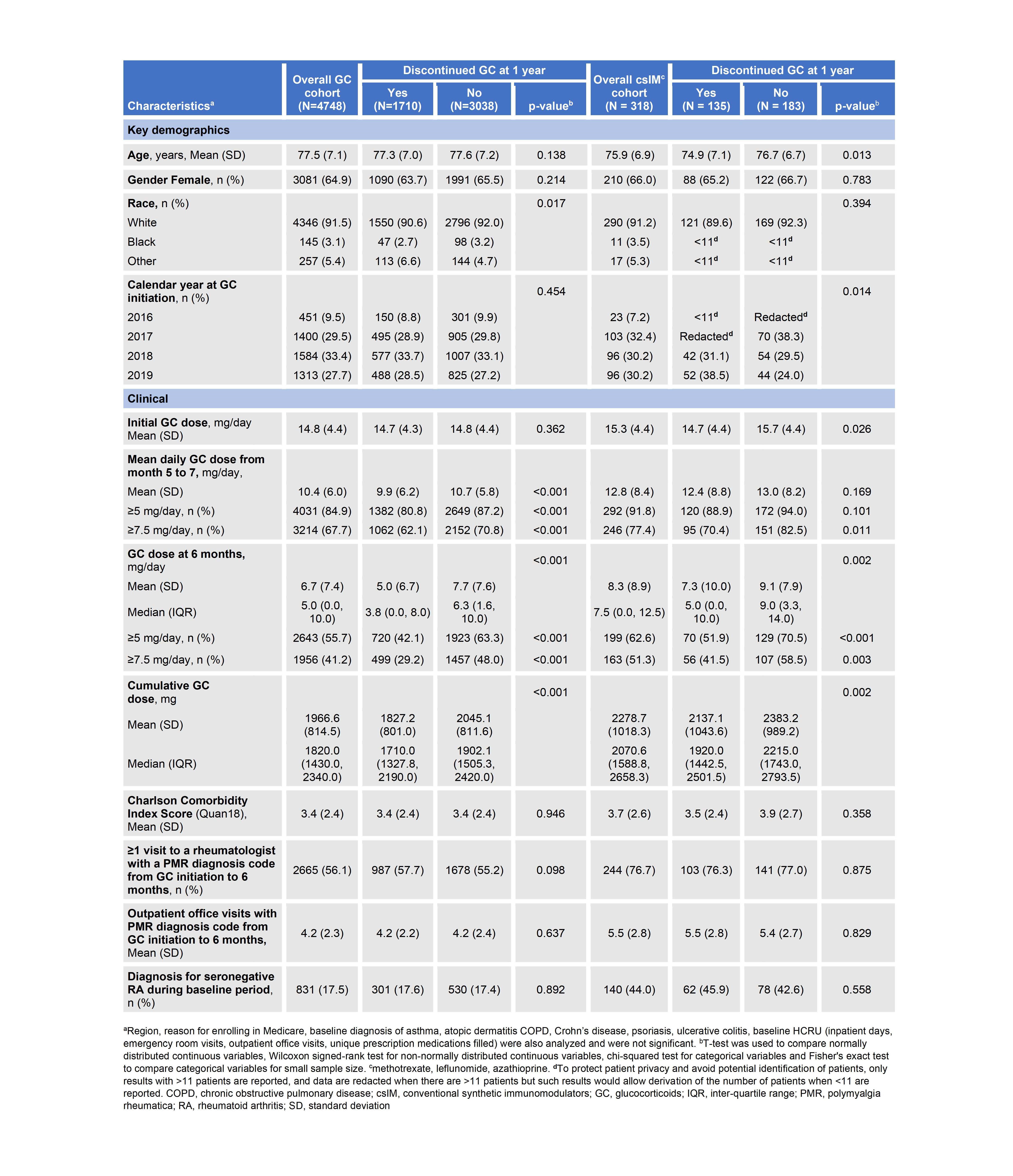
Results: A total of 4,748 patients were included in the GC cohort and 318 in the csIM cohort. MTX was the most commonly used csIM [200/318 (62.9%)]. Of patients in the GC cohort and csIM cohort, 3,038 (64.0%) and 183 (57.5%) were on GC at 1 year, respectively. In both GC and csIM cohorts, significantly more patients on GC vs. off GC at 1 year were on GC dose ≥5 mg at 6 months. Patients on GC vs. off GC at 1 year also had significantly higher cumulative GC use at 6 months in both cohorts (Table 1).
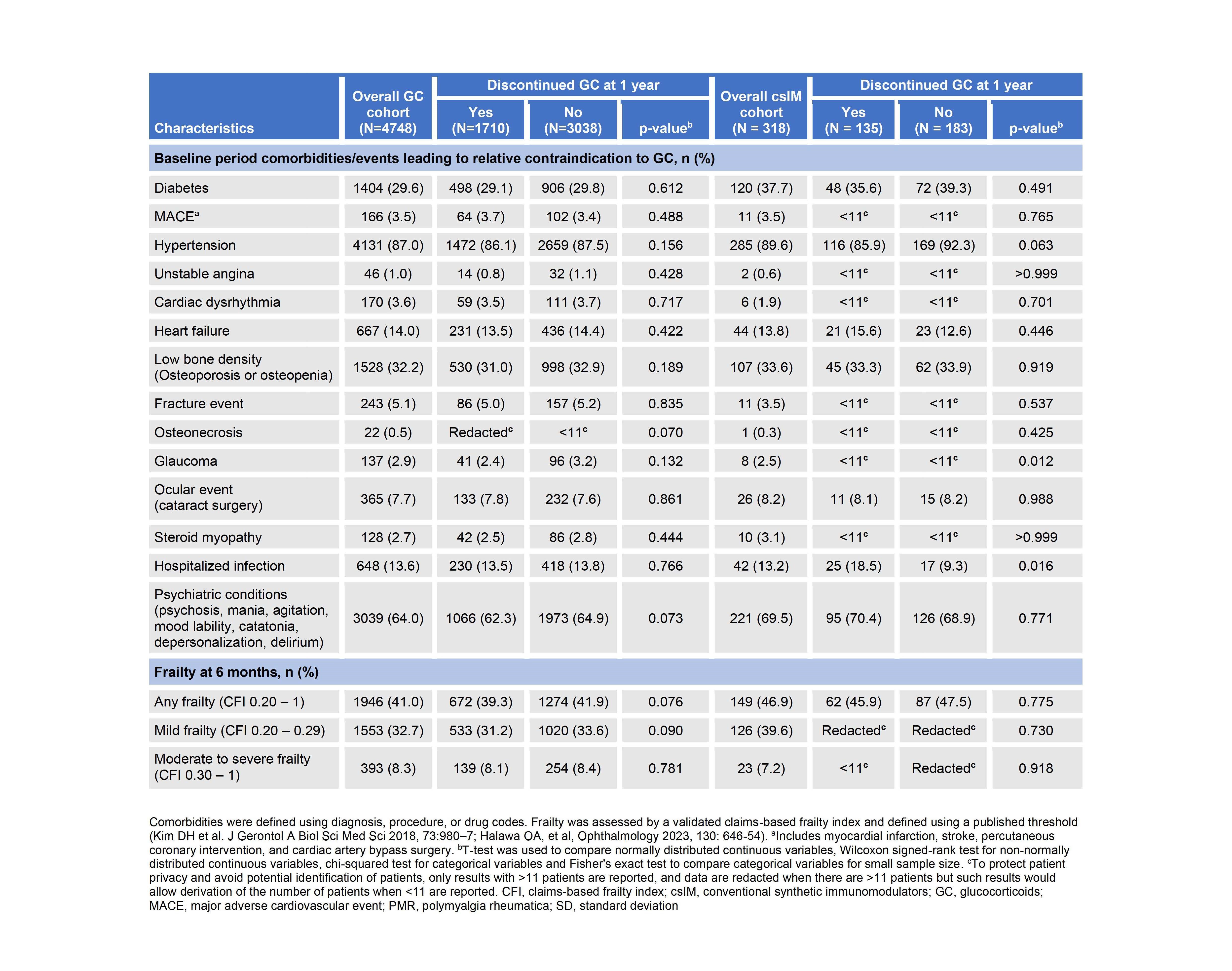
In the GC cohort, demographic characteristics and presence of comorbidities or frailty at 6 months, were not associated with GC use at 1 year while in the csIM cohort, age, year GC was initiated, initial GC dose, and glaucoma were significantly associated with GC use at 1 year (Tables 1,2). A sensitivity analysis including patients who initiated MTX within 6 months found 55% (111/200) of patients were on GC at 1 year and MTX use was significantly associated with GC use at 1 year: fewer patients on GC vs. off GC at 1 year had received MTX within 6 months [111/3149 (3.5%) vs 89/1799 (4.9%); p=0.015].
Conclusion: More than half of PMR patients remained on GC beyond 1 year. Evaluation of GC dose at 6 months may be useful to identify patients who may benefit from GC sparing therapy.
Original presentation: EULAR 2024.
To cite this abstract in AMA style:
Dua A, Rubbert-Roth A, Ford K, Fiore S, Araujo L, Beukelman T, Xie F, Curtis J. Characteristics Associated with Long-Term Glucocorticoids Use in Patients with New Onset Polymyalgia Rheumatica [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/characteristics-associated-with-long-term-glucocorticoids-use-in-patients-with-new-onset-polymyalgia-rheumatica/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-associated-with-long-term-glucocorticoids-use-in-patients-with-new-onset-polymyalgia-rheumatica/