Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Accounting for the integration of new immunosuppressive treatment options (belimumab and voclosporin) since 2021, we aimed to describe real-world treatment patterns in patients with lupus nephritis (LN) overall and by their demographics.
Methods: This retrospective observational cohort study used healthcare administrative claims data from the IQVIA PharMetrics® Plus Database from January 1, 2017, to September 30, 2023 (study period). Adults with a diagnosis of LN were identified if they had ≥1 inpatient or ≥2 outpatient claims ≥30 days apart with an International Classification of Diseases, 10th Revision, Clinical Modifications code M32.14 from January 1, 2018, to March 31, 2023 (identification period). The index date was the date of first LN diagnosis. Patients were required to have continuous enrollment in the 12-month pre-index and 6-month post-index periods, and no LN diagnosis in the pre-index baseline period. Patients with a claim for renal transplant, dialysis or end-stage renal disease in the pre-index period, or a claim for any malignancy, HIV, hepatitis B or C or tuberculosis in the study period, were excluded. Time to initial treatment following index LN diagnosis was assessed and initial treatment regimen identified and described according to index diagnosis date (before or on/after January 1, 2021). Treatment received within 30 days of first identified LN treatment was considered as the initial regimen.
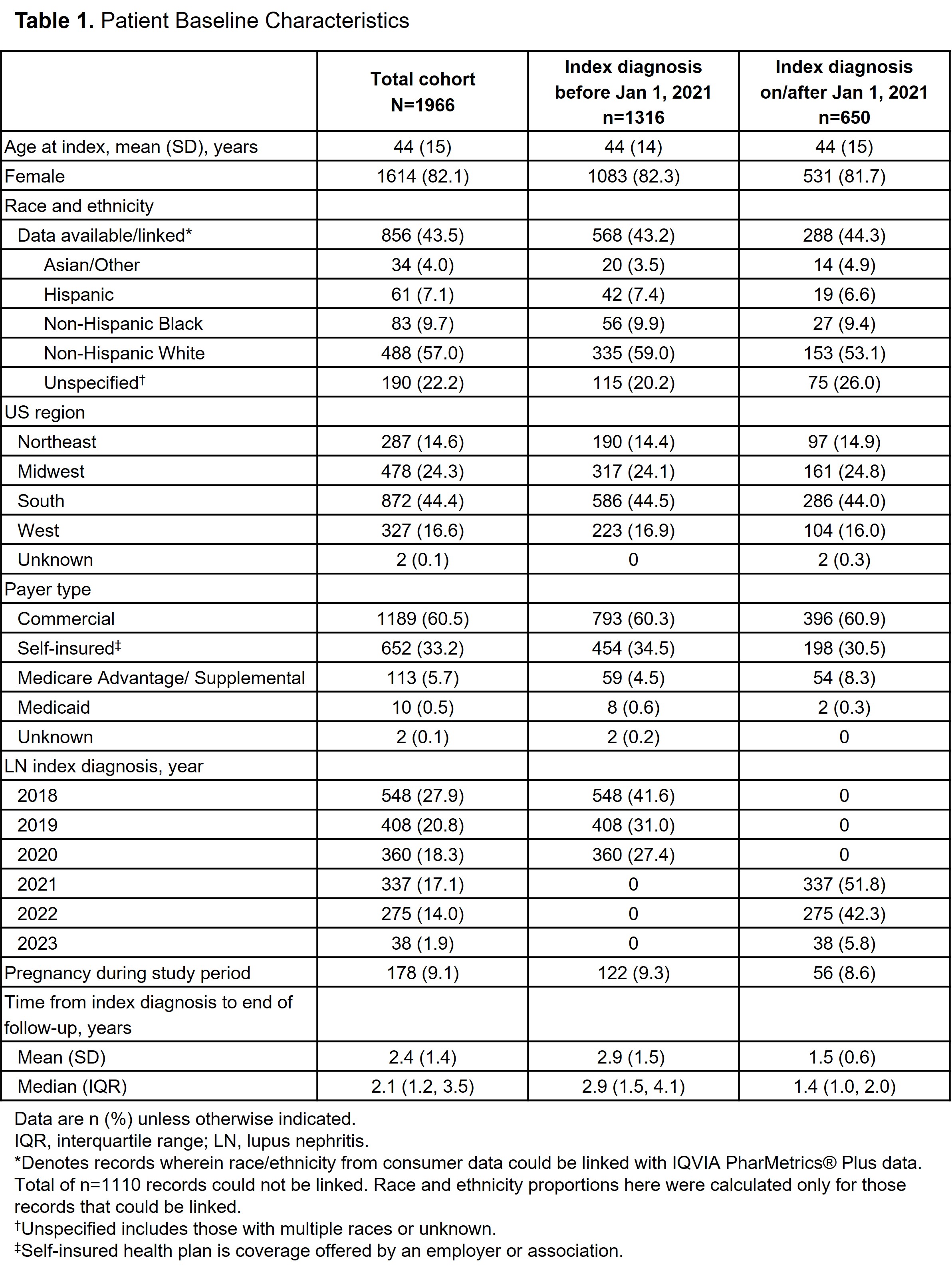
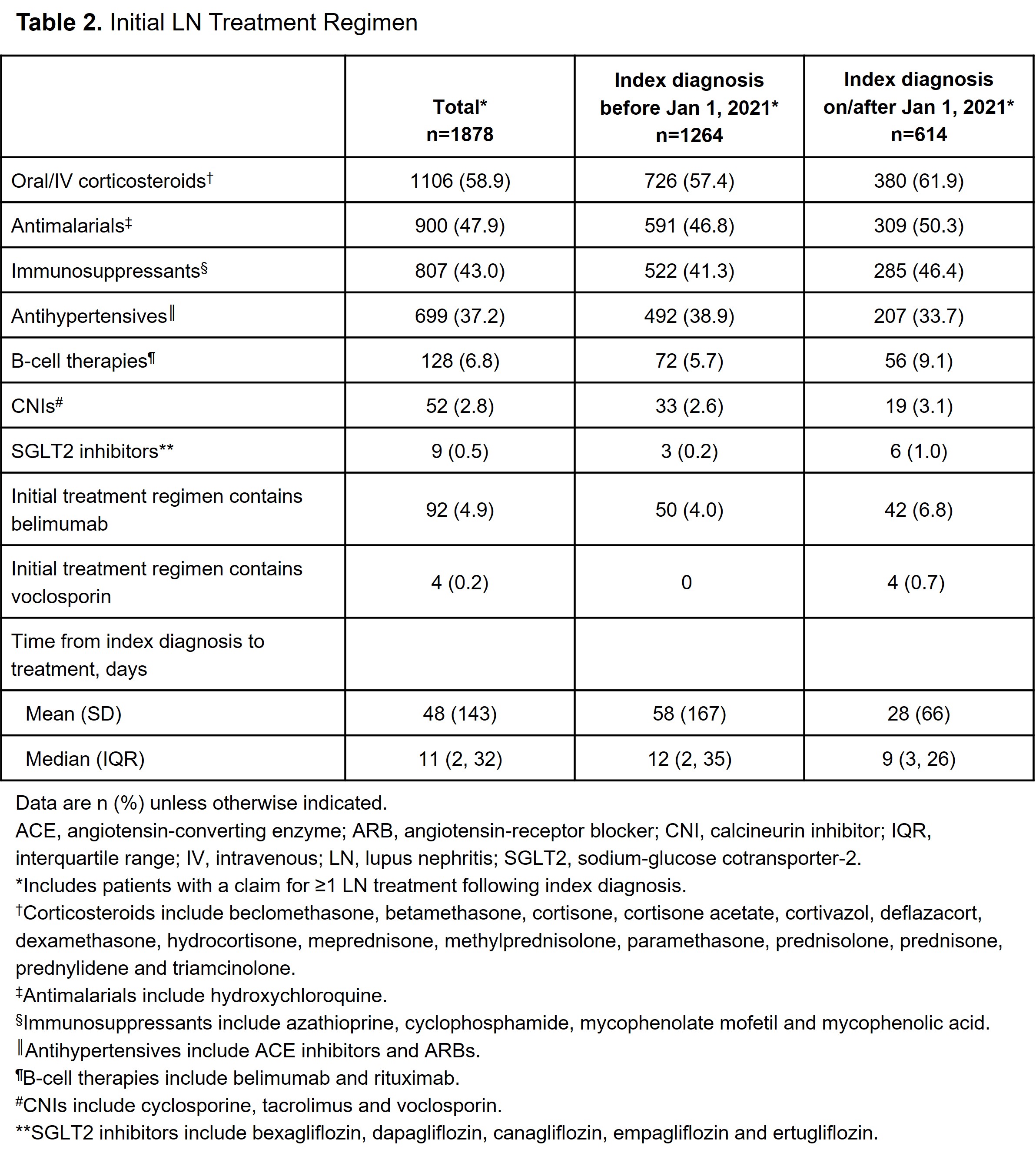
Results: A total of 1966 patients with LN were identified. Mean (SD) age was 44 (15) years, 82.1% were female, 60.5% had commercial insurance and 44.4% were from the South US region. The average follow-up was 2.4 years post-index LN diagnosis, and 9.1% had ≥1 pregnancy code in the study period. Index LN diagnosis occurred in 66.9% of patients before and 33.1% on/after January 1, 2021. Among patients who had a record of ≥1 LN treatment following index diagnosis (n=1878), the initial treatment regimen included ≥1 of the following: oral/intravenous corticosteroids (n=1106; 58.9%), antimalarials (n=900; 47.9%), immunosuppressants including mycophenolate mofetil (n=807; 43.0%), antihypertensives (n=699; 37.2%), B-cell therapies including belimumab (n=128; 6.8%), calcineurin inhibitors including voclosporin (n=52; 2.8%) and sodium-glucose cotransporter-2 inhibitors (n=9; 0.5%). Median time from index diagnosis to initial LN treatment was 11 days, with shorter time from diagnosis to treatment observed for patients diagnosed on/after vs before January 1, 2021 (median, 9 vs 12 days, respectively). Patient characteristics were similar for index diagnosis occurring before and after 2021; however, differences were noted in the initial treatments (Tables 1 and 2).
Conclusion: Time from index LN diagnosis to treatment was shorter for patients diagnosed on/after January 1, 2021, than those diagnosed before, suggesting a potential movement toward earlier treatment in recent years. This will be explored for specific treatments in future analyses. Further work will investigate LN treatment patterns beyond the initial regimen including line of therapy and switching, as well as treatment in relation to the updated KDIGO 2024 guidelines and by patients’ sociodemographic factors.
To cite this abstract in AMA style:
Patel A, Ng C, Lindsay L, Xia Z, Pendergraft III W, Dall'Era M. Characteristics and Treatment Patterns of Patients with Lupus Nephritis: A Retrospective Claims Database Study in the USA [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/characteristics-and-treatment-patterns-of-patients-with-lupus-nephritis-a-retrospective-claims-database-study-in-the-usa/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-and-treatment-patterns-of-patients-with-lupus-nephritis-a-retrospective-claims-database-study-in-the-usa/